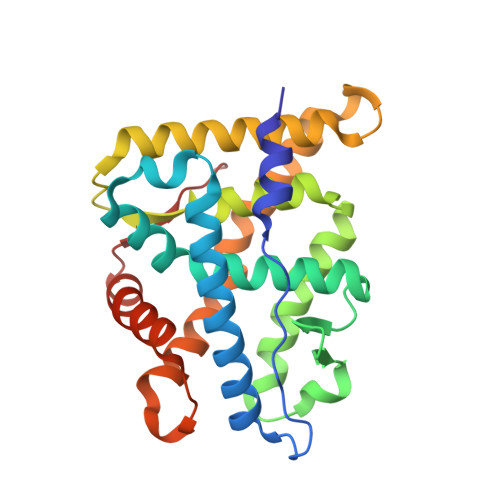
Disruption of a key ligand-H-bond network drives dissociative properties in vamorolone for Duchenne muscular dystrophy treatment.
Liu, X., Wang, Y., Gutierrez, J.S., Damsker, J.M., Nagaraju, K., Hoffman, E.P., Ortlund, E.A.(2020) Proc Natl Acad Sci U S A 117: 24285-24293
- PubMed: 32917814
- DOI: https://doi.org/10.1073/pnas.2006890117
- Primary Citation of Related Structures:
6W9K, 6W9L, 6W9M - PubMed Abstract:
Duchenne muscular dystrophy is a genetic disorder that shows chronic and progressive damage to skeletal and cardiac muscle leading to premature death. Antiinflammatory corticosteroids targeting the glucocorticoid receptor (GR) are the current standard of care but drive adverse side effects such as deleterious bone loss. Through subtle modification to a steroidal backbone, a recently developed drug, vamorolone, appears to preserve beneficial efficacy but with significantly reduced side effects. We use combined structural, biophysical, and biochemical approaches to show that loss of a receptor-ligand hydrogen bond drives these remarkable therapeutic effects. Moreover, vamorolone uniformly weakens coactivator associations but not corepressor associations, implicating partial agonism as the main driver of its dissociative properties. Additionally, we identify a critical and evolutionarily conserved intramolecular network connecting the ligand to the coregulator binding surface. Interruption of this allosteric network by vamorolone selectively reduces GR-driven transactivation while leaving transrepression intact. Our results establish a mechanistic understanding of how vamorolone reduces side effects, guiding the future design of partial agonists as selective GR modulators with an improved therapeutic index.
- Department of Biochemistry, Emory University, Atlanta, GA 30322.
Organizational Affiliation: