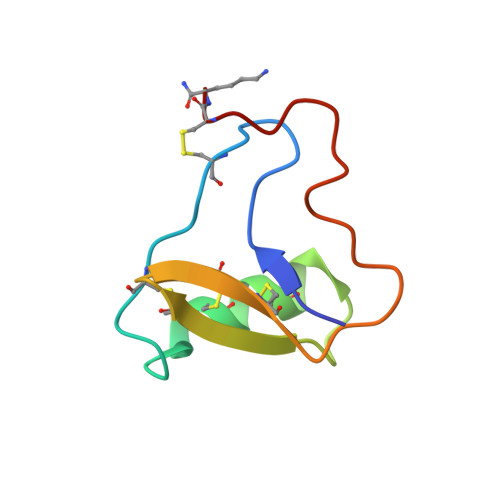
The three-dimensional structure of the toxic peptide Cl13 from the scorpion Centruroides limpidus.
Lopez-Giraldo, A.E., Olamendi-Portugal, T., Riano-Umbarila, L., Becerril, B., Possani, L.D., Delepierre, M., Del Rio-Portilla, F.(2020) Toxicon 184: 158-166
- PubMed: 32569846
- DOI: https://doi.org/10.1016/j.toxicon.2020.06.011
- Primary Citation of Related Structures:
6VXW - PubMed Abstract:
Cl13 is a toxin purified previously from the venom of the Mexican scorpion Centruroides limpidus. This toxin affects the function of voltage gated Na + -channels, human subtypes Nav1.4, Nav1.5 and Nav1.6 in a similar manner as other known β-toxins from scorpion venoms. Here, we report a correction of the primary structure of Cl13, previously published. The peptide does contain 66 amino acids, but residue 58 is a tryptophan and the last C-terminal amino acid is an amidated lysine, instead of arginine. The main contribution of this communication is the determination of the 3D-structure of Cl13, by solution NMR, showing that Cl13 has the classical cysteine-stabilized α/β (CSα/β) folding. It has a triple stranded antiparallel beta sheet commonly present in scorpion sodium channel β-toxins. In addition, we report and discuss a comparison of Cl13 structure with two other toxins (Cn2 and Css2) from scorpions of the same genus Centruroides, which shows important surface similarities with the structure reported here. Finally, the lack of neutralization of Cl13 toxin by two single-chain antibody fragments (scFvs), named LR and 10FG2, which are capable of neutralizing various toxins from Mexican scorpions, is revised. In particular, 10FG2 is capable of neutralizing toxins Cll1 and Cll2 of the same scorpion C. limpidus. The reasons why LR and 10FG2 are unable of neutralizing Cl13 toxin are discussed.
- Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Ciudad Universitaria, CdMx, 04510, Mexico.
Organizational Affiliation: