Molecular basis of diverse substrate recognition by EspG5 chaperone from mycobacterial ESX-5 Type VII Secretion System.
Williamson, Z.A., Korotkova, N., Korotkov, K.V.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Currently 6VJ5 does not have a validation slider image.
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| PE-PGRS family protein PE25 | 101 | Mycobacterium tuberculosis H37Rv | Mutation(s): 0 Gene Names: PE25, Rv2431c, LH57_13295 | 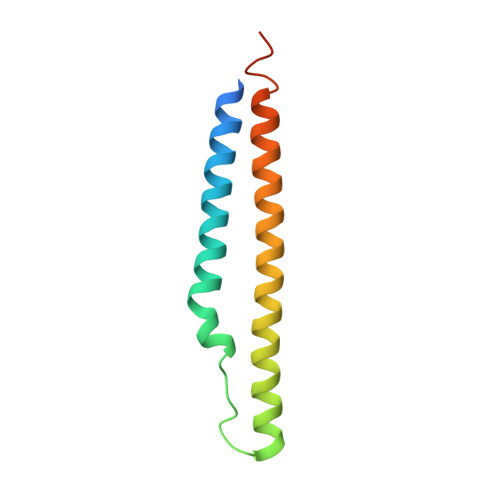 | |
UniProt | |||||
Find proteins for I6X486 (Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)) Explore I6X486 Go to UniProtKB: I6X486 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | I6X486 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| PPE family protein PPE41 | 194 | Mycobacterium tuberculosis H37Rv | Mutation(s): 1 Gene Names: PPE41, Rv2430c, LH57_13290 | 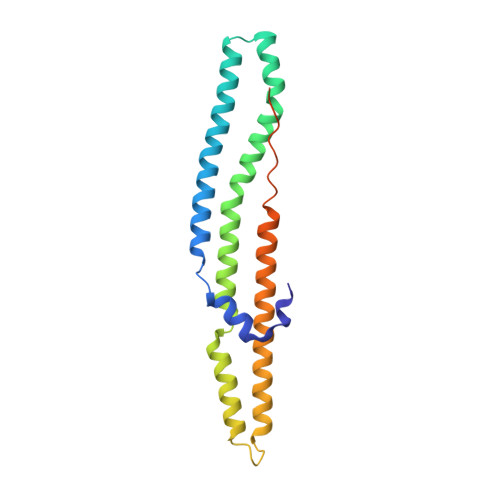 | |
UniProt | |||||
Find proteins for Q79FE1 (Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)) Explore Q79FE1 Go to UniProtKB: Q79FE1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q79FE1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ESX-5 secretion-associated protein EspG5 | 300 | Mycobacterium marinum M | Mutation(s): 0 Gene Names: espG5, MMAR_2676 | 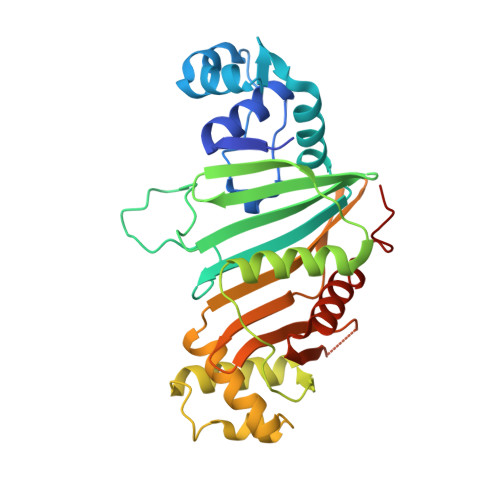 | |
UniProt | |||||
Find proteins for B2HSU5 (Mycobacterium marinum (strain ATCC BAA-535 / M)) Explore B2HSU5 Go to UniProtKB: B2HSU5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B2HSU5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CL Query on CL | D [auth B], E [auth B] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 139.02 | α = 90 |
| b = 139.02 | β = 90 |
| c = 170.57 | γ = 120 |
| Software Name | Purpose |
|---|---|
| XSCALE | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| PHENIX | phasing |
Currently 6VJ5 does not have a validation slider image.
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | R01AI119022 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | P20GM103486 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | P30GM110787 |