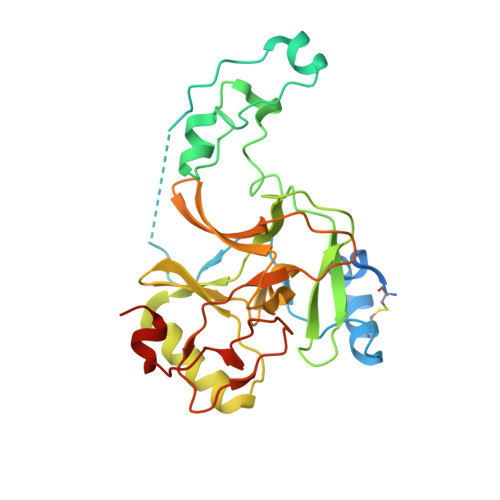
Sequence specificity analysis of the SETD2 protein lysine methyltransferase and discovery of a SETD2 super-substrate.
Schuhmacher, M.K., Beldar, S., Khella, M.S., Brohm, A., Ludwig, J., Tempel, W., Weirich, S., Min, J., Jeltsch, A.(2020) Commun Biol 3: 511-511
- PubMed: 32939018
- DOI: https://doi.org/10.1038/s42003-020-01223-6
- Primary Citation of Related Structures:
6VDB - PubMed Abstract:
SETD2 catalyzes methylation at lysine 36 of histone H3 and it has many disease connections. We investigated the substrate sequence specificity of SETD2 and identified nine additional peptide and one protein (FBN1) substrates. Our data showed that SETD2 strongly prefers amino acids different from those in the H3K36 sequence at several positions of its specificity profile. Based on this, we designed an optimized super-substrate containing four amino acid exchanges and show by quantitative methylation assays with SETD2 that the super-substrate peptide is methylated about 290-fold more efficiently than the H3K36 peptide. Protein methylation studies confirmed very strong SETD2 methylation of the super-substrate in vitro and in cells. We solved the structure of SETD2 with bound super-substrate peptide containing a target lysine to methionine mutation, which revealed better interactions involving three of the substituted residues. Our data illustrate that substrate sequence design can strongly increase the activity of protein lysine methyltransferases.
- Institute of Biochemistry and Technical Biochemistry, University of Stuttgart, Allmandring 31, 70569, Stuttgart, Germany.
Organizational Affiliation: