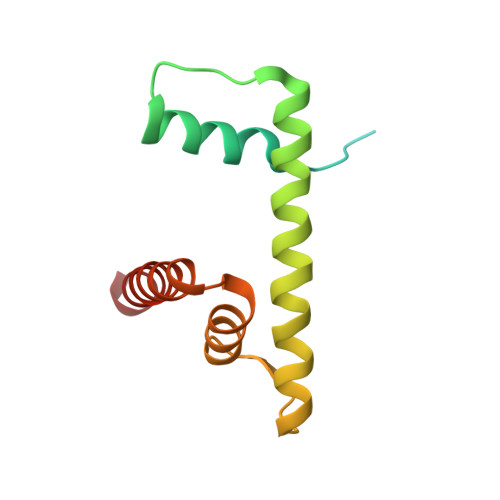
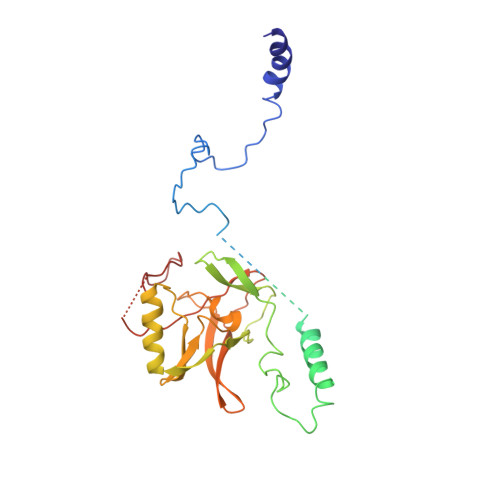
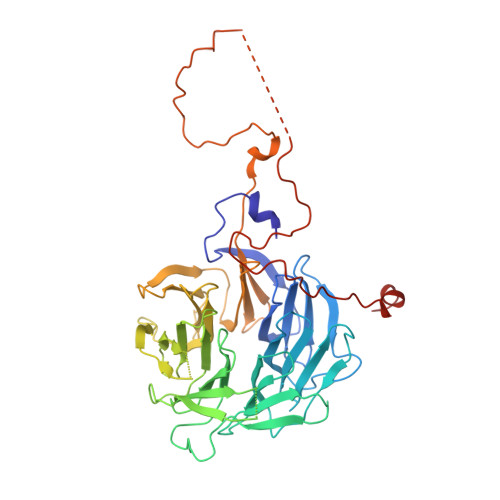
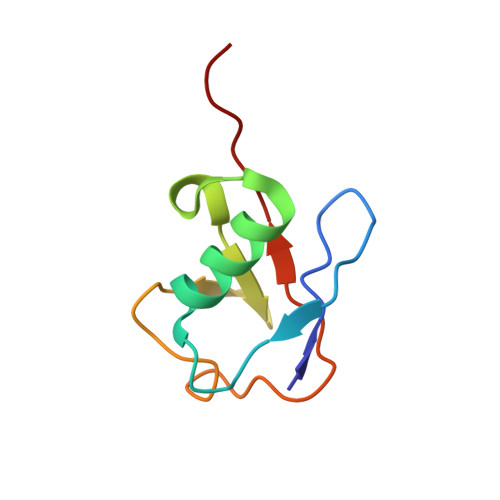
Structural Basis of H2B Ubiquitination-Dependent H3K4 Methylation by COMPASS.
Hsu, P.L., Shi, H., Leonen, C., Kang, J., Chatterjee, C., Zheng, N.(2019) Mol Cell 76: 712
- PubMed: 31733991
- DOI: https://doi.org/10.1016/j.molcel.2019.10.013
- Primary Citation of Related Structures:
6UGM, 6UH5 - PubMed Abstract:
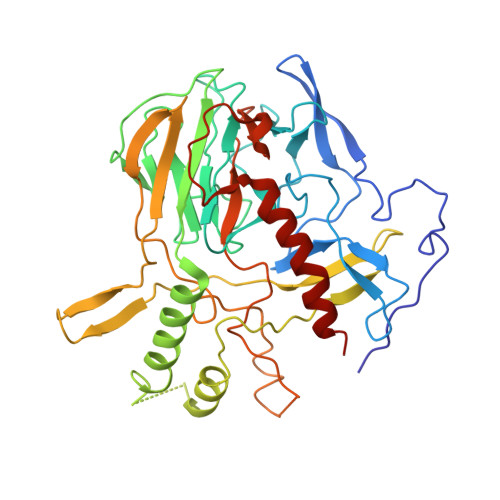
The COMPASS (complex of proteins associated with Set1) complex represents the prototype of the SET1/MLL family of methyltransferases that controls gene transcription by H3K4 methylation (H3K4me). Although H2B monoubiquitination (H2Bub) is well known as a prerequisite histone mark for COMPASS activity, how H2Bub activates COMPASS remains unclear. Here, we report the cryoelectron microscopy (cryo-EM) structures of an extended COMPASS catalytic module (CM) bound to the H2Bub and free nucleosome. The COMPASS CM clamps onto the nucleosome disk-face via an extensive interface to capture the flexible H3 N-terminal tail. The interface also sandwiches a critical Set1 arginine-rich motif (ARM) that autoinhibits COMPASS. Unexpectedly, without enhancing COMPASS-nucleosome interaction, H2Bub activates the enzymatic assembly by packing against Swd1 and alleviating the inhibitory effect of the Set1 ARM upon fastening it to the acidic patch. By delineating the spatial configuration of the COMPASS-H2Bub-nucleosome assembly, our studies establish the structural framework for understanding the long-studied H2Bub-H3K4me histone modification crosstalk.
- Department of Pharmacology, Box 357280, University of Washington, Seattle, WA 98195, USA; Howard Hughes Medical Institute, University of Washington, Seattle, WA 98195, USA.
Organizational Affiliation: