Scaffold Simplification Strategy Leads to a Novel Generation of Dual Human Immunodeficiency Virus and Enterovirus-A71 Entry Inhibitors.
Martinez-Gualda, B., Sun, L., Marti-Mari, O., Noppen, S., Abdelnabi, R., Bator, C.M., Quesada, E., Delang, L., Mirabelli, C., Lee, H., Schols, D., Neyts, J., Hafenstein, S., Camarasa, M.J., Gago, F., San-Felix, A.(2020) J Med Chem 63: 349-368
- PubMed: 31809045
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01737
- Primary Citation of Related Structures:
6UH1, 6UH6, 6UH7 - PubMed Abstract:
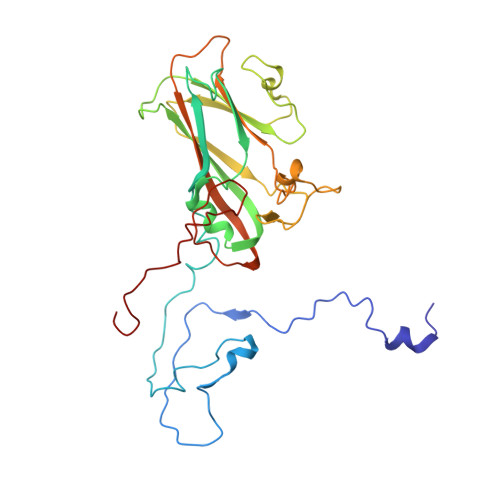
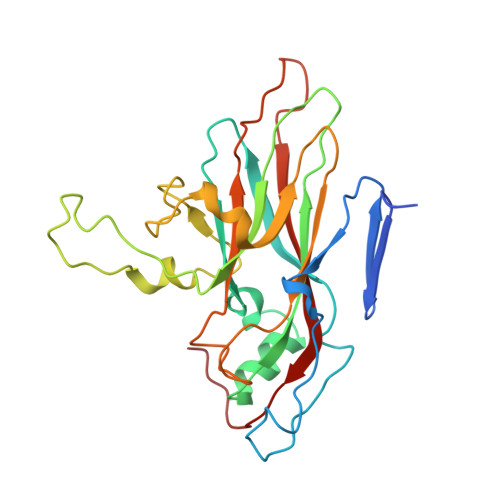
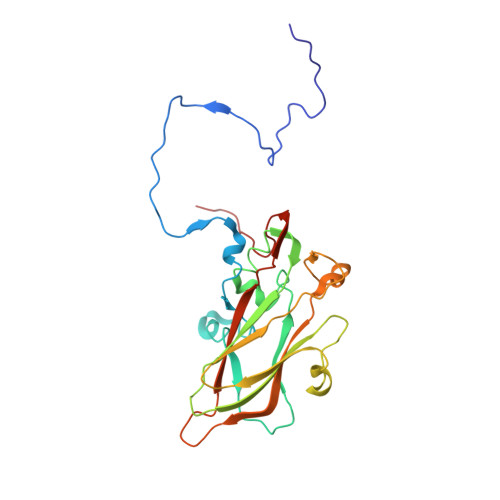
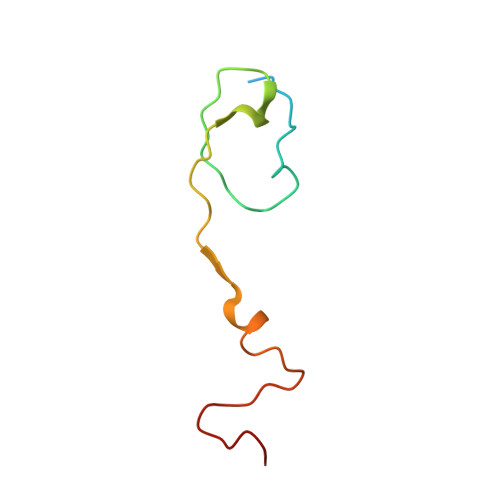
Currently, there are only three FDA-approved drugs that inhibit human immunodeficiency virus (HIV) entry-fusion into host cells. The situation is even worse for enterovirus EV71 infection for which no antiviral therapies are available. We describe here the discovery of potent entry dual inhibitors of HIV and EV71. These compounds contain in their structure three or four tryptophan (Trp) residues linked to a central scaffold. Critical for anti-HIV/EV71 activity is the presence of extra phenyl rings, bearing one or two carboxylates, at the C2 position of the indole ring of each Trp residue. The most potent derivatives, 22 and 30 , inhibit early steps of the replicative cycles of HIV-1 and EV-A71 by interacting with their respective viral surfaces (glycoprotein gp120 of HIV and the fivefold axis of the EV-A71 capsid). The high potency, low toxicity, facile chemical synthesis, and great opportunities for chemical optimization make them useful prototypes for future medicinal chemistry studies.
- Instituto de Química Médica (IQM-CSIC) , 28006 Madrid , Spain.
Organizational Affiliation: