Ketosteroid isomerase (C. testosteroni) with truncated & designed loop for precise positioning of a catalytic E38
Kundert, K., Thompson, M.C., Liu, L., Fraser, J.S., Kortemme, T.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ketosteroid isomerase with truncated and designed loop | 124 | Comamonas testosteroni | Mutation(s): 0 EC: 5.3.3.1 | 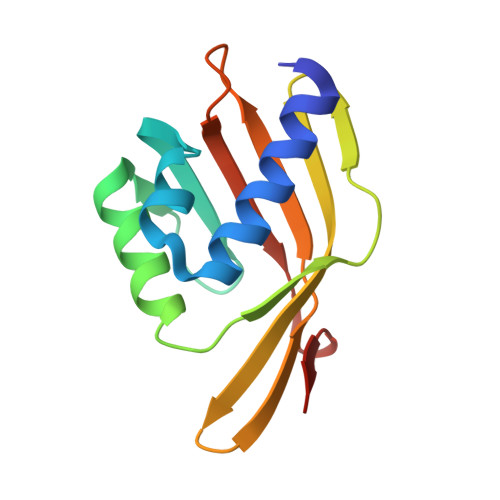 | |
UniProt | |||||
Find proteins for P00947 (Comamonas testosteroni) Explore P00947 Go to UniProtKB: P00947 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00947 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| DXC Query on DXC | B [auth A], C [auth A] | (3ALPHA,5BETA,12ALPHA)-3,12-DIHYDROXYCHOLAN-24-OIC ACID C24 H40 O4 KXGVEGMKQFWNSR-LLQZFEROSA-N |  | ||
| PO4 Query on PO4 | D [auth A], E [auth A] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 53.15 | α = 90 |
| b = 53.15 | β = 90 |
| c = 178.03 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| xia2 | data reduction |
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R01-GM110089 |
| National Science Foundation (NSF, United States) | United States | DBI-1564692 |