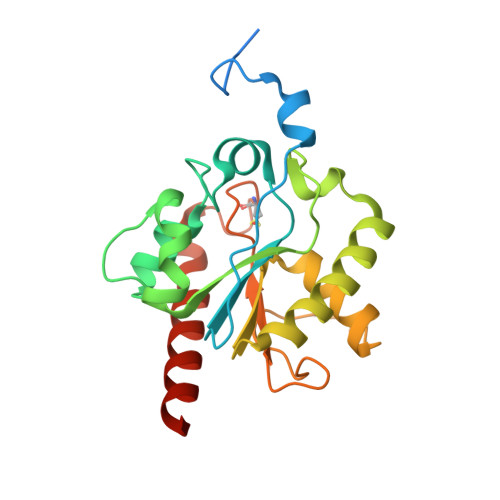
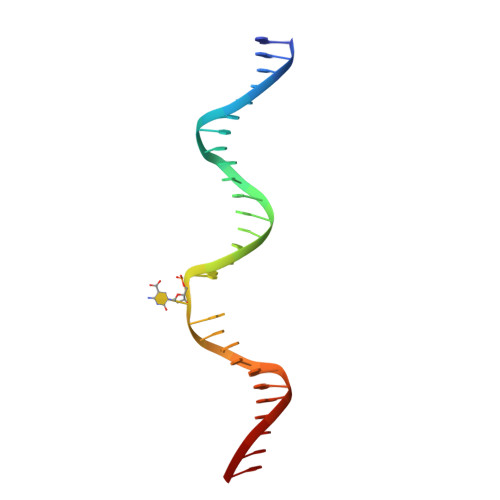
Excision of 5-Carboxylcytosine by Thymine DNA Glycosylase.
Pidugu, L.S., Dai, Q., Malik, S.S., Pozharski, E., Drohat, A.C.(2019) J Am Chem Soc 141: 18851-18861
- PubMed: 31693361
- DOI: https://doi.org/10.1021/jacs.9b10376
- Primary Citation of Related Structures:
6U15, 6U16, 6U17 - PubMed Abstract:
5-Methylcytosine (mC) is an epigenetic mark that is written by methyltransferases, erased through passive and active mechanisms, and impacts transcription, development, diseases including cancer, and aging. Active DNA demethylation involves TET-mediated stepwise oxidation of mC to 5-hydroxymethylcytosine, 5-formylcytosine (fC), or 5-carboxylcytosine (caC), excision of fC or caC by thymine DNA glycosylase (TDG), and subsequent base excision repair. Many elements of this essential process are poorly defined, including TDG excision of caC. To address this problem, we solved high-resolution structures of human TDG bound to DNA with cadC (5-carboxyl-2'-deoxycytidine) flipped into its active site. The structures unveil detailed enzyme-substrate interactions that mediate recognition and removal of caC, many involving water molecules. Importantly, two water molecules contact a carboxylate oxygen of caC and are poised to facilitate acid-catalyzed caC excision. Moreover, a substrate-dependent conformational change in TDG modulates the hydrogen bond interactions for one of these waters, enabling productive interaction with caC. An Asn residue (N191) that is critical for caC excision is found to contact N3 and N4 of caC, suggesting a mechanism for acid-catalyzed base excision that features an N3-protonated form of caC but would be ineffective for C, mC, or hmC. We also investigated another Asn residue (N140) that is catalytically essential and strictly conserved in the TDG-MUG enzyme family. A structure of N140A-TDG bound to cadC DNA provides the first high-resolution insight into how enzyme-substrate interactions, including water molecules, are impacted by depleting the conserved Asn, informing its role in binding and addition of the nucleophilic water molecule.
- Department of Biochemistry and Molecular Biology , University of Maryland School of Medicine , Baltimore , Maryland 21201 , United States.
Organizational Affiliation: