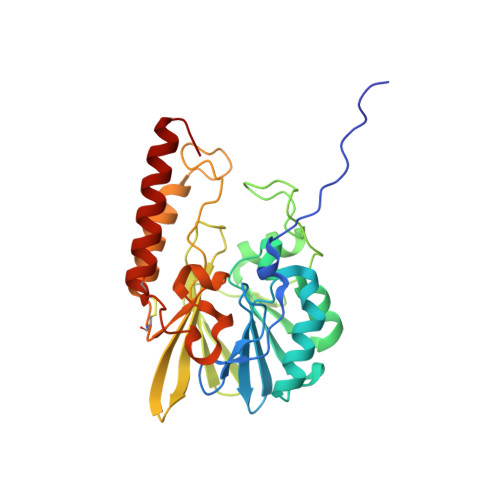
Crystal Structure of the metallo-beta-lactamase L1 from Stenotrophomonas maltophilia in the complex with the inhibitor captopril.
Kim, Y., Maltseva, N., Endres, M., Joachimiak, A., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.