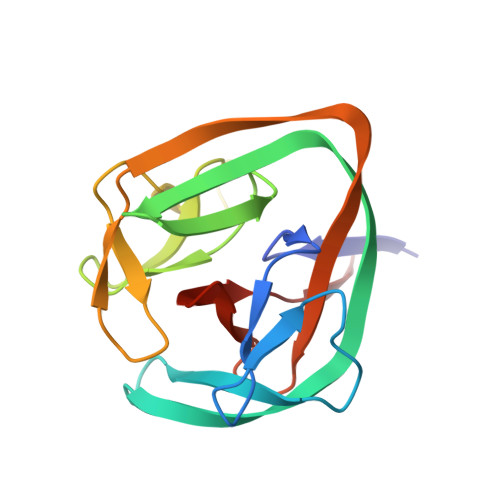
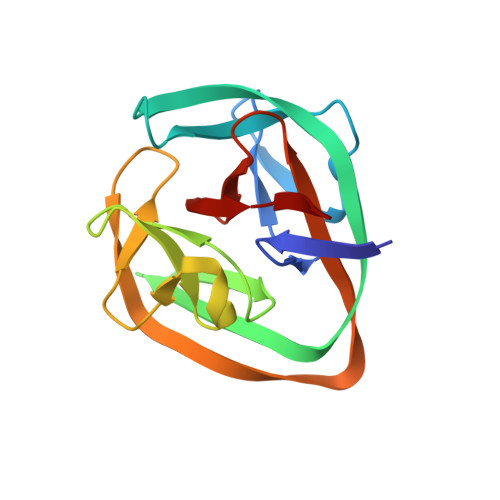
Structural basis of ubiquitination mediated by protein splicing in early Eukarya.
Chiarini, V., Fiorillo, A., Camerini, S., Crescenzi, M., Nakamura, S., Battista, T., Guidoni, L., Colotti, G., Ilari, A.(2021) Biochim Biophys Acta Gen Subj 1865: 129844-129844
- PubMed: 33444728
- DOI: https://doi.org/10.1016/j.bbagen.2021.129844
- Primary Citation of Related Structures:
6TMM, 6Y75 - PubMed Abstract:
Inteins are intervening proteins, which are known to perform protein splicing. The reaction results in the production of an intein domain and an inteinless protein, which shows no trace of the insertion. BIL2 is part of the polyubiquitin locus of Tetrahymena thermophila (BUBL), where two bacterial-intein-like (BIL) domains lacking the C + 1 nucleophile, are flanked by two independent ubiquitin-like domains (ubl4/ubl5). We solved the X-ray structures of BIL2 in both the inactive and unprecedented, zinc-induced active, forms. Then, we characterized by mass spectrometry the BUBL splicing products in the absence and in the presence of T.thRas-GTPase. Finally, we investigated the effect of ubiquitination on T.thRas-GTPase by molecular dynamics simulations. The structural analysis demonstrated that zinc-induced conformational change activates protein splicing. Moreover, mass spectrometry characterization of the splicing products shed light on the possible function of BIL2, which operates as a "single-ubiquitin-dispensing-platform", allowing the conjugation, via isopeptide bond formation (K(εNH 2 )-C-ter), of ubl4 to either ubl5 or T.thRas-GTPase. Lastly, we demonstrated that T.thRas-GTPase ubiquitination occurs in proximity of the nucleotide binding pocket and stabilizes the protein active state. We demonstrated that BIL2 is activated by zinc and that protein splicing induced by this intein does not take place through classical or aminolysis mechanisms but via formation of a covalent isopeptide bond, causing the ubiquitination of endogenous substrates such as T.thRas-GTPase. In this "enzyme-free" ubiquitination mechanism the isopeptide formation, which canonically requires E1-E2-E3 enzymatic cascade and constitutes the alphabet of ubiquitin biology, is achieved in a single, concerted step without energy consumption.
- Program in Structural Biology and Biophysics, Institute of Biotechnology, University of Helsinki, Viikinkaari 1, P.O. Box 65, FI-00014 Helsinki, Finland.
Organizational Affiliation: