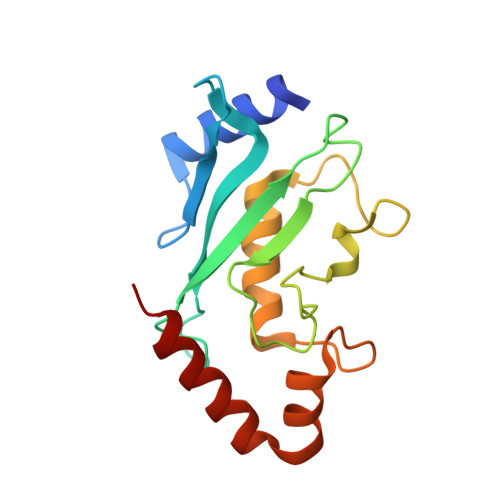
Lysine acylation using conjugating enzymes for site-specific modification and ubiquitination of recombinant proteins.
Hofmann, R., Akimoto, G., Wucherpfennig, T.G., Zeymer, C., Bode, J.W.(2020) Nat Chem 12: 1008-1015
- PubMed: 32929246
- DOI: https://doi.org/10.1038/s41557-020-0528-y
- Primary Citation of Related Structures:
6SYF - PubMed Abstract:
Enzymes are powerful tools for protein labelling due to their specificity and mild reaction conditions. Many protocols, however, are restricted to modifications at protein termini, rely on non-peptidic metabolites or require large recognition domains. Here we report a chemoenzymatic method, which we call lysine acylation using conjugating enzymes (LACE), to site-specifically modify folded proteins at internal lysine residues. LACE relies on a minimal genetically encoded tag (four residues) recognized by the E2 small ubiquitin-like modifier-conjugating enzyme Ubc9, and peptide or protein thioesters. Together, this approach obviates the need for E1 and E3 enzymes, enabling isopeptide formation with just Ubc9 in a programmable manner. We demonstrate the utility of LACE by the site-specific attachment of biochemical probes, one-pot dual-labelling in combination with sortase, and the conjugation of wild-type ubiquitin and ISG15 to recombinant proteins.
- Laboratorium für Organische Chemie, Department of Chemistry and Applied Biosciences, ETH Zürich, Zürich, Switzerland.
Organizational Affiliation: