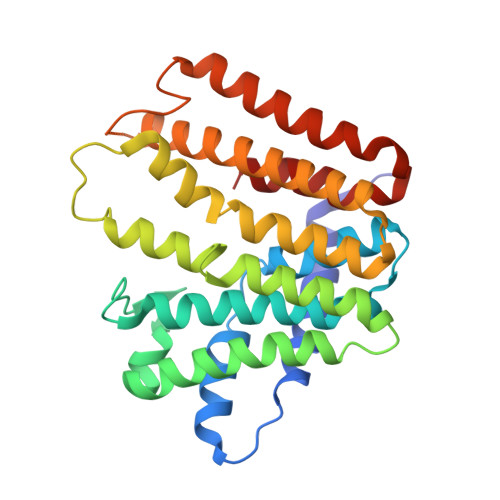
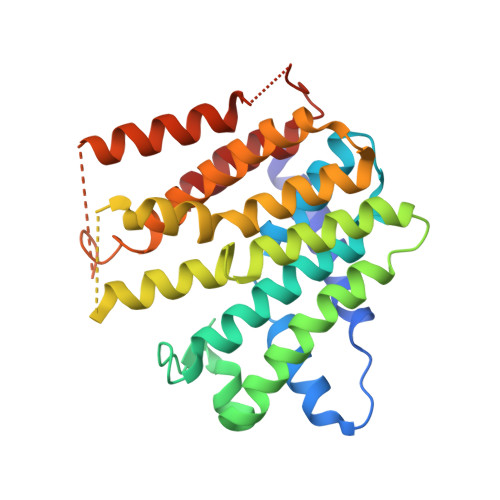
Crystal Structure of Geranylgeranyl Pyrophosphate Synthase (CrtE) Involved in Cyanobacterial Terpenoid Biosynthesis.
Feng, Y., Morgan, R.M.L., Fraser, P.D., Hellgardt, K., Nixon, P.J.(2020) Front Plant Sci 11: 589-589
- PubMed: 32523588
- DOI: https://doi.org/10.3389/fpls.2020.00589
- Primary Citation of Related Structures:
6SXL, 6SXN - PubMed Abstract:
Cyanobacteria are photosynthetic prokaryotes that perform oxygenic photosynthesis. Due to their ability to use the photon energy of sunlight to fix carbon dioxide into biomass, cyanobacteria are promising hosts for the sustainable production of terpenoids, also known as isoprenoids, a diverse class of natural products with potential as advanced biofuels and high-value chemicals. However, the cyanobacterial enzymes involved in the biosynthesis of the terpene precursors needed to make more complicated terpenoids are poorly characterized. Here we show that the predicted type II prenyltransferase CrtE encoded by the model cyanobacterium Synechococcus sp. PCC 7002 is homodimeric and able to synthesize C20-geranylgeranyl pyrophosphate (GGPP) from C5-isopentenyl pyrophosphate (IPP) and C5-dimethylallyl pyrophosphate (DMAPP). The crystal structure of CrtE solved to a resolution of 2.7 Å revealed a strong structural similarity to the large subunit of the heterodimeric geranylgeranyl pyrophosphate synthase 1 from Arabidopsis thaliana with each subunit containing 14 helices. Using mutagenesis, we confirmed that the fourth and fifth amino acids (Met-87 and Ser-88) before the first conserved aspartate-rich motif (FARM) play important roles in controlling chain elongation. While the WT enzyme specifically produced GGPP, variants M87F and S88Y could only generate C15-farnesyl pyrophosphate (FPP), indicating that residues with large side chains obstruct product elongation. In contrast, replacement of M87 with the smaller Ala residue allowed the formation of the longer C25-geranylfarnesyl pyrophosphate (GFPP) product. Overall, our results provide new structural and functional information on the cyanobacterial CrtE enzyme that could lead to the development of improved cyanobacterial platforms for terpenoid production.
- Department of Chemical Engineering, Imperial College London, London, United Kingdom.
Organizational Affiliation: