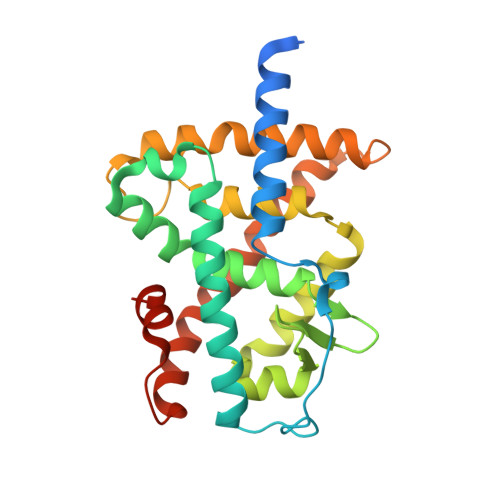
Regulation of RXR-RAR Heterodimers by RXR- and RAR-Specific Ligands and Their Combinations.
le Maire, A., Teyssier, C., Balaguer, P., Bourguet, W., Germain, P.(2019) Cells 8
- PubMed: 31694317
- DOI: https://doi.org/10.3390/cells8111392
- Primary Citation of Related Structures:
6SSQ, 6STI - PubMed Abstract:
The three subtypes (α, β, and γ) of the retinoic acid receptor (RAR) are ligand-dependent transcription factors that mediate retinoic acid signaling by forming heterodimers with the retinoid X receptor (RXR). Heterodimers are functional units that bind ligands (retinoids), transcriptional co-regulators and DNA, to regulate gene networks controlling cell growth, differentiation, and death. Using biochemical, crystallographic, and cellular approaches, we have set out to explore the spectrum of possibilities to regulate RXR-RAR heterodimer-dependent transcription through various pharmacological classes of RAR- and RXR- specific ligands, alone or in combination. We reveal the molecular details by which these compounds direct specificity and functionality of RXR-RAR heterodimers. Among these ligands, we have reevaluated and improved the molecular and structural definition of compounds CD2665, Ro41-5253, LE135, or LG100754, highlighting novel functional features of these molecules. Our analysis reveals a model of RXR-RAR heterodimer action in which each subunit retains its intrinsic properties in terms of ligand and co-regulator binding. However, their interplay upon the combined action of RAR- and RXR-ligands allows for the fine tuning of heterodimer activity. It also stresses the importance of accurate ligand characterization to use synthetic selective retinoids appropriately and avoid data misinterpretations.
- Centre de Biochimie Structurale (CBS), CNRS, INSERM, University of Montpellier, 34090 Montpellier, France.
Organizational Affiliation: