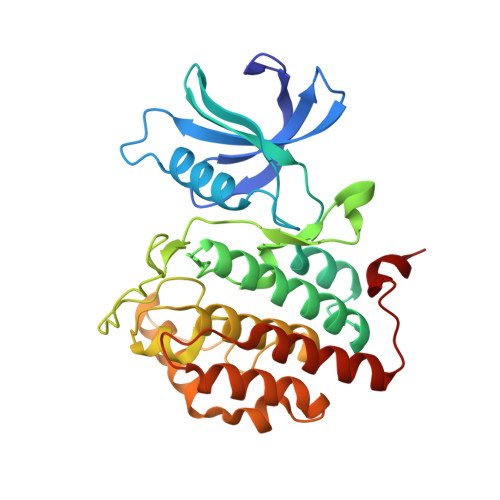
p63 uses a switch-like mechanism to set the threshold for induction of apoptosis.
Gebel, J., Tuppi, M., Chaikuad, A., Hotte, K., Schroder, M., Schulz, L., Lohr, F., Gutfreund, N., Finke, F., Henrich, E., Mezhyrova, J., Lehnert, R., Pampaloni, F., Hummer, G., Stelzer, E.H.K., Knapp, S., Dotsch, V.(2020) Nat Chem Biol 16: 1078-1086
- PubMed: 32719556
- DOI: https://doi.org/10.1038/s41589-020-0600-3
- Primary Citation of Related Structures:
6RU6, 6RU7, 6RU8 - PubMed Abstract:
The p53 homolog TAp63α is the transcriptional key regulator of genome integrity in oocytes. After DNA damage, TAp63α is activated by multistep phosphorylation involving multiple phosphorylation events by the kinase CK1, which triggers the transition from a dimeric and inactive conformation to an open and active tetramer that initiates apoptosis. By measuring activation kinetics in ovaries and single-site phosphorylation kinetics in vitro with peptides and full-length protein, we show that TAp63α phosphorylation follows a biphasic behavior. Although the first two CK1 phosphorylation events are fast, the third one, which constitutes the decisive step to form the active conformation, is slow. Structure determination of CK1 in complex with differently phosphorylated peptides reveals the structural mechanism for the difference in the kinetic behavior based on an unusual CK1/TAp63α substrate interaction in which the product of one phosphorylation step acts as an inhibitor for the following one.
- Institute of Biophysical Chemistry and Center for Biomolecular Magnetic Resonance and Cluster of Excellence Macromolecular Complexes (CEF), Goethe University, Frankfurt am Main, Germany.
Organizational Affiliation: