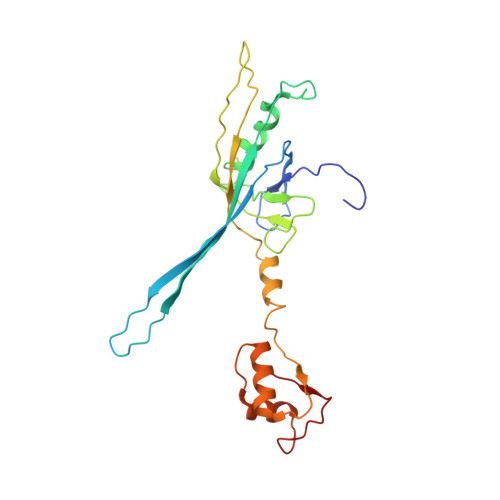
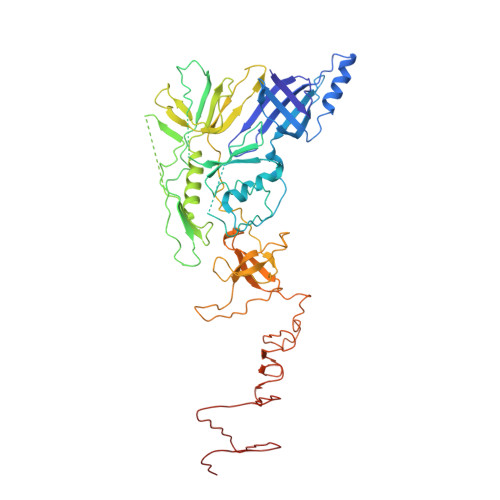
Atomic structures of an entire contractile injection system in both the extended and contracted states.
Desfosses, A., Venugopal, H., Joshi, T., Felix, J., Jessop, M., Jeong, H., Hyun, J., Heymann, J.B., Hurst, M.R.H., Gutsche, I., Mitra, A.K.(2019) Nat Microbiol 4: 1885-1894
- PubMed: 31384001
- DOI: https://doi.org/10.1038/s41564-019-0530-6
- Primary Citation of Related Structures:
6RAO, 6RAP, 6RBK, 6RBN, 6RC8, 6RGL - PubMed Abstract:
Contractile injection systems are sophisticated multiprotein nanomachines that puncture target cell membranes. Although the number of atomic-resolution insights into contractile bacteriophage tails, bacterial type six secretion systems and R-pyocins is rapidly increasing, structural information on the contraction of bacterial phage-like protein-translocation structures directed towards eukaryotic hosts is scarce. Here, we characterize the antifeeding prophage AFP from Serratia entomophila by cryo-electron microscopy. We present the high-resolution structure of the entire AFP particle in the extended state, trace 11 protein chains de novo from the apical cap to the needle tip, describe localization variants and perform specific structural comparisons with related systems. We analyse inter-subunit interactions and highlight their universal conservation within contractile injection systems while revealing the specificities of AFP. Furthermore, we provide the structure of the AFP sheath-baseplate complex in a contracted state. This study reveals atomic details of interaction networks that accompany and define the contraction mechanism of toxin-delivery tailocins, offering a comprehensive framework for understanding their mode of action and for their possible adaptation as biocontrol agents.
- School of Biological Sciences, University of Auckland, Auckland, New Zealand.
Organizational Affiliation: