Structural insights into the activity and regulation of human Josephin-2
Grasty, K.C., Weeks, S.D., Loll, P.J.(2019) J Struct Biol 3: 100011
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2019) J Struct Biol 3: 100011
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Josephin-2 | 188 | Homo sapiens | Mutation(s): 0 Gene Names: JOSD2, SBBI54 EC: 3.4.19.12 | 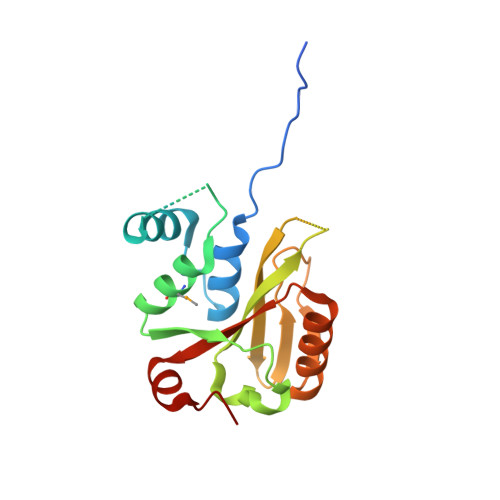 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8TAC2 (Homo sapiens) Explore Q8TAC2 Go to UniProtKB: Q8TAC2 | |||||
PHAROS: Q8TAC2 GTEx: ENSG00000161677 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8TAC2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Polyubiquitin-B | 75 | Homo sapiens | Mutation(s): 0 Gene Names: UBB | 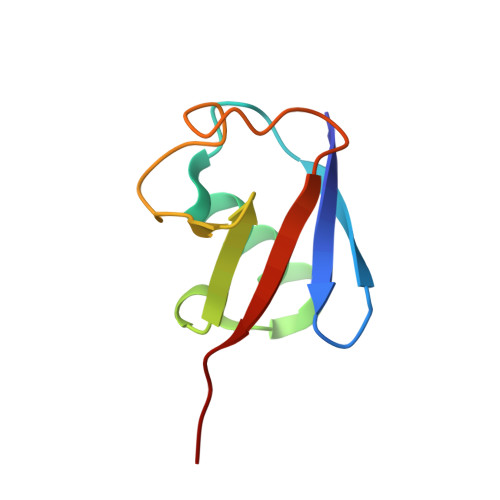 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0CG47 (Homo sapiens) Explore P0CG47 Go to UniProtKB: P0CG47 | |||||
PHAROS: P0CG47 GTEx: ENSG00000170315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0CG47 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NEH Query on NEH | C [auth B] | ETHANAMINE C2 H7 N QUSNBJAOOMFDIB-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 102.12 | α = 90 |
| b = 102.12 | β = 90 |
| c = 92.2 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) | United States | R01NS065140 |