Structural and functional characterization of the mitochondrial complex IV assembly factor Coa6.
Maghool, S., Cooray, N.D.G., Stroud, D.A., Aragao, D., Ryan, M.T., Maher, M.J.(2019) Life Sci Alliance 2
- PubMed: 31515291
- DOI: https://doi.org/10.26508/lsa.201900458
- Primary Citation of Related Structures:
6PCE, 6PCF - PubMed Abstract:
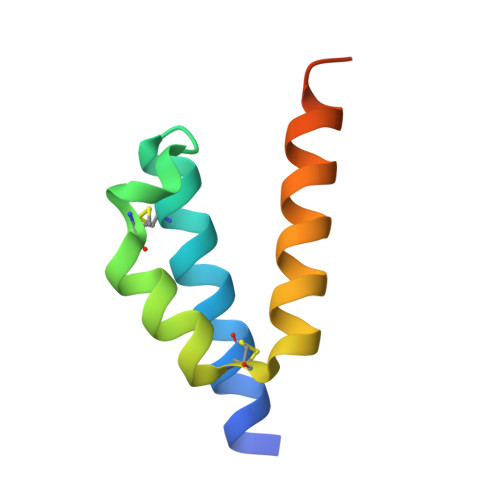
Assembly factors play key roles in the biogenesis of many multi-subunit protein complexes regulating their stability, activity, and the incorporation of essential cofactors. The human assembly factor Coa6 participates in the biogenesis of the Cu A site in complex IV (cytochrome c oxidase, COX). Patients with mutations in Coa6 suffer from mitochondrial disease due to complex IV deficiency. Here, we present the crystal structures of human Coa6 and the pathogenic W59C Coa6-mutant protein. These structures show that Coa6 has a 3-helical bundle structure, with the first 2 helices tethered by disulfide bonds, one of which likely provides the copper-binding site. Disulfide-mediated oligomerization of the W59C Coa6 protein provides a structural explanation for the loss-of-function mutation.
- Department of Biochemistry and Genetics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Australia.
Organizational Affiliation: