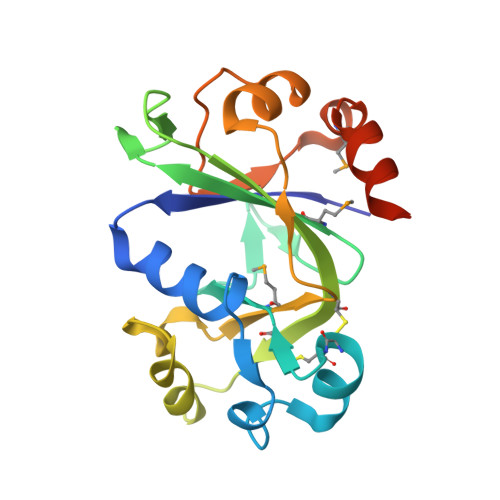
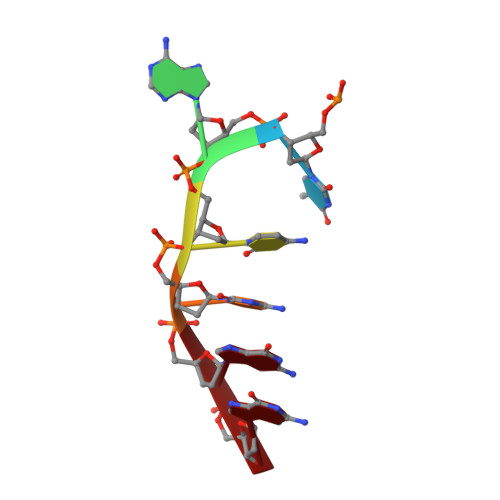
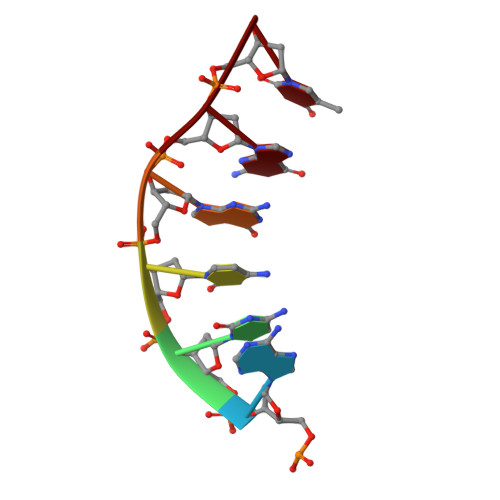
The structure of theThermococcus gammatoleransMcrB N-terminal domain reveals a new mode of substrate recognition and specificity among McrB homologs.
Hosford, C.J., Bui, A.Q., Chappie, J.S.(2020) J Biological Chem 295: 743-756
- PubMed: 31822563
- DOI: https://doi.org/10.1074/jbc.RA119.010188
- Primary Citation of Related Structures:
6P0F, 6P0G - PubMed Abstract:
McrBC is a two-component, modification-dependent restriction system that cleaves foreign DNA-containing methylated cytosines. Previous crystallographic studies have shown that Escherichia coli McrB uses a base-flipping mechanism to recognize these modified substrates with high affinity. The side chains stabilizing both the flipped base and the distorted duplex are poorly conserved among McrB homologs, suggesting that other mechanisms may exist for binding modified DNA. Here we present the structures of the Thermococcus gammatolerans McrB DNA-binding domain (TgΔ185) both alone and in complex with a methylated DNA substrate at 1.68 and 2.27 Å resolution, respectively. The structures reveal that TgΔ185 consists of a YT521-B homology (YTH) domain, which is commonly found in eukaryotic proteins that bind methylated RNA and is structurally unrelated to the E. coli McrB DNA-binding domain. Structural superposition and co-crystallization further show that TgΔ185 shares a conserved aromatic cage with other YTH domains, which forms the binding pocket for a flipped-out base. Mutational analysis of this aromatic cage supports its role in conferring specificity for the methylated adenines, whereas an extended basic surface present in TgΔ185 facilitates its preferential binding to duplex DNA rather than RNA. Together, these findings establish a new binding mode and specificity among McrB homologs and expand the biological roles of YTH domains.
- Department of Molecular Medicine, Cornell University, Ithaca, New York 14853.
Organizational Affiliation: