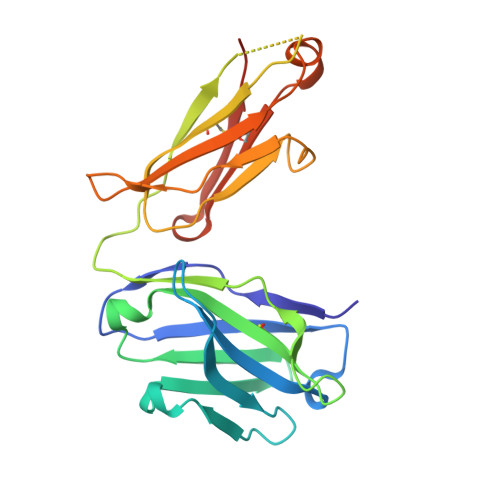
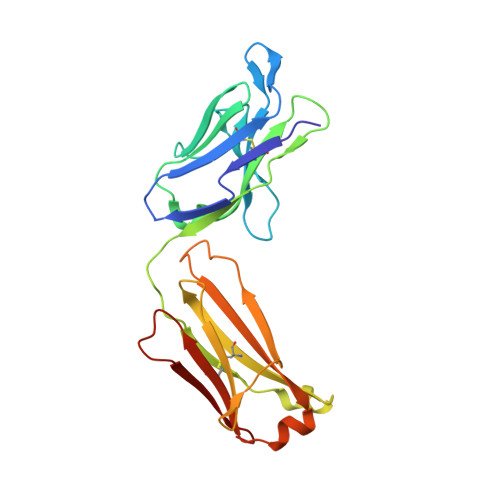
Development of tibulizumab, a tetravalent bispecific antibody targeting BAFF and IL-17A for the treatment of autoimmune disease.
Benschop, R.J., Chow, C.K., Tian, Y., Nelson, J., Barmettler, B., Atwell, S., Clawson, D., Chai, Q., Jones, B., Fitchett, J., Torgerson, S., Ji, Y., Bina, H., Hu, N., Ghanem, M., Manetta, J., Wroblewski, V.J., Lu, J., Allan, B.W.(2019) MAbs 11: 1175-1190
- PubMed: 31181988
- DOI: https://doi.org/10.1080/19420862.2019.1624463
- Primary Citation of Related Structures:
6NOU, 6NOV - PubMed Abstract:
We describe a bispecific dual-antagonist antibody against human B cell activating factor (BAFF) and interleukin 17A (IL-17). An anti-IL-17 single-chain variable fragment (scFv) derived from ixekizumab (Taltz®) was fused via a glycine-rich linker to anti-BAFF tabalumab. The IgG-scFv bound both BAFF and IL-17 simultaneously with identical stoichiometry as the parental mAbs. Stability studies of the initial IgG-scFv revealed chemical degradation and aggregation not observed in either parental antibody. The anti-IL-17 scFv showed a high melting temperature ( Tm ) by differential scanning calorimetry (73.1°C), but also concentration-dependent, initially reversible, protein self-association. To engineer scFv stability, three parallel approaches were taken: labile complementary-determining region (CDR) residues were replaced by stable, affinity-neutral amino acids, CDR charge distribution was balanced, and a H44-L100 interface disulfide bond was introduced. The Tm of the disulfide-stabilized scFv was largely unperturbed, yet it remained monodispersed at high protein concentration. Fluorescent dye binding titrations indicated reduced solvent exposure of hydrophobic residues and decreased proteolytic susceptibility was observed, both indicative of enhanced conformational stability. Superimposition of the H44-L100 scFv (PDB id: 6NOU) and ixekizumab antigen-binding fragment (PDB id: 6NOV) crystal structures revealed nearly identical orientation of the frameworks and CDR loops. The stabilized bispecific molecule LY3090106 (tibulizumab) potently antagonized both BAFF and IL-17 in cell-based and in vivo mouse models. In cynomolgus monkey, it suppressed B cell development and survival and remained functionally intact in circulation, with a prolonged half-life. In summary, we engineered a potent bispecific antibody targeting two key cytokines involved in human autoimmunity amenable to clinical development.
- a Biotechnology Discovery Research, Lilly Research Laboratories, Eli Lilly and Company Corporate Center , Indianapolis , IN , USA.
Organizational Affiliation: