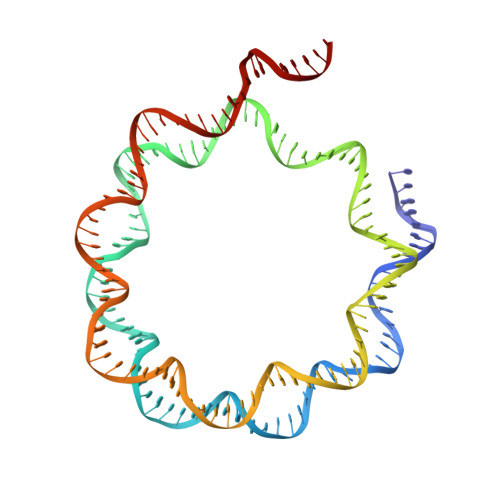
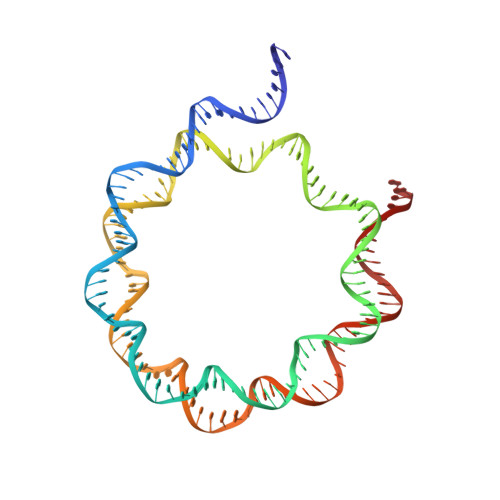
Cryo-EM structures of remodeler-nucleosome intermediates suggest allosteric control through the nucleosome.
Armache, J.P., Gamarra, N., Johnson, S.L., Leonard, J.D., Wu, S., Narlikar, G.J., Cheng, Y.(2019) Elife 8
- PubMed: 31210637
- DOI: https://doi.org/10.7554/eLife.46057
- Primary Citation of Related Structures:
6NE3 - PubMed Abstract:
The SNF2h remodeler slides nucleosomes most efficiently as a dimer, yet how the two protomers avoid a tug-of-war is unclear. Furthermore, SNF2h couples histone octamer deformation to nucleosome sliding, but the underlying structural basis remains unknown. Here we present cryo-EM structures of SNF2h-nucleosome complexes with ADP-BeF x that capture two potential reaction intermediates. In one structure, histone residues near the dyad and in the H2A-H2B acidic patch, distal to the active SNF2h protomer, appear disordered. The disordered acidic patch is expected to inhibit the second SNF2h protomer, while disorder near the dyad is expected to promote DNA translocation. The other structure doesn't show octamer deformation, but surprisingly shows a 2 bp translocation. FRET studies indicate that ADP-BeF x predisposes SNF2h-nucleosome complexes for an elemental translocation step. We propose a model for allosteric control through the nucleosome, where one SNF2h protomer promotes asymmetric octamer deformation to inhibit the second protomer, while stimulating directional DNA translocation.
- Department of Biochemistry and Biophysics, University of California, San Francisco, San Francisco, United States.
Organizational Affiliation: