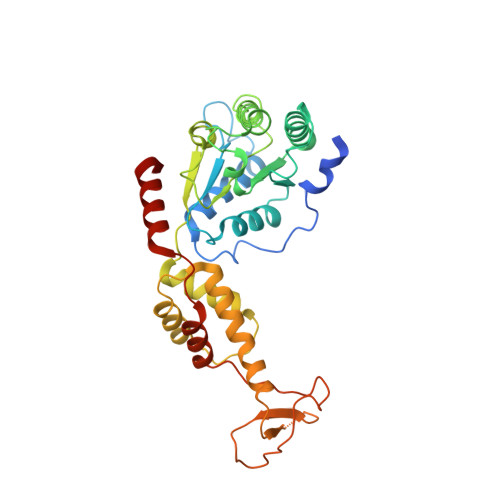
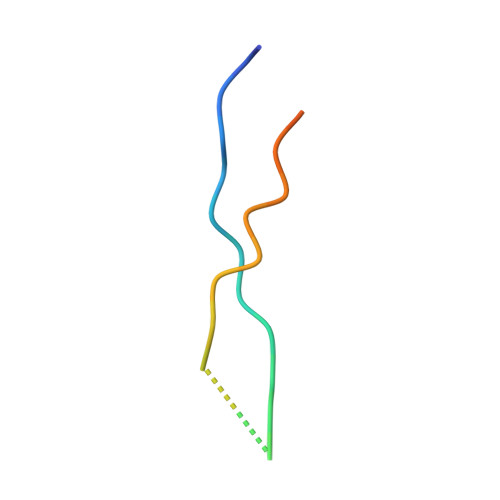
Structure of Vps4 with circular peptides and implications for translocation of two polypeptide chains by AAA+ ATPases.
Han, H., Fulcher, J.M., Dandey, V.P., Iwasa, J.H., Sundquist, W.I., Kay, M.S., Shen, P.S., Hill, C.P.(2019) Elife 8
- PubMed: 31184588
- DOI: https://doi.org/10.7554/eLife.44071
- Primary Citation of Related Structures:
6NDY, 6OO2 - PubMed Abstract:
Many AAA+ ATPases form hexamers that unfold protein substrates by translocating them through their central pore. Multiple structures have shown how a helical assembly of subunits binds a single strand of substrate, and indicate that translocation results from the ATP-driven movement of subunits from one end of the helical assembly to the other end. To understand how more complex substrates are bound and translocated, we demonstrated that linear and cyclic versions of peptides bind to the S. cerevisiae AAA+ ATPase Vps4 with similar affinities, and determined cryo-EM structures of cyclic peptide complexes. The peptides bind in a hairpin conformation, with one primary strand equivalent to the single chain peptide ligands, while the second strand returns through the translocation pore without making intimate contacts with Vps4. These observations indicate a general mechanism by which AAA+ ATPases may translocate a variety of substrates that include extended chains, hairpins, and crosslinked polypeptide chains.
- Department of Biochemistry, University of Utah, Salt Lake City, United States.
Organizational Affiliation: