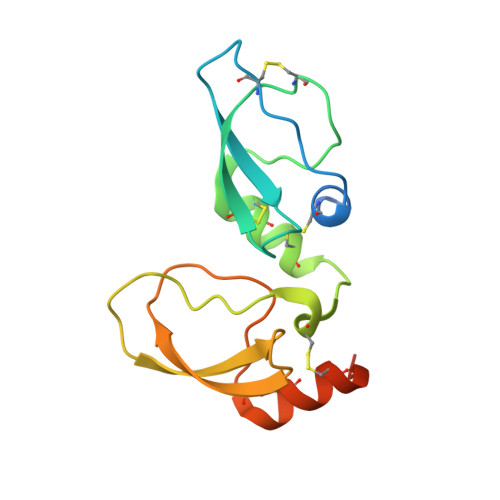
NMR structure determination of Ixolaris and factor X(a) interaction reveals a noncanonical mechanism of Kunitz inhibition.
De Paula, V.S., Sgourakis, N.G., Francischetti, I.M.B., Almeida, F.C.L., Monteiro, R.Q., Valente, A.P.(2019) Blood 134: 699-708
- PubMed: 31133602
- DOI: https://doi.org/10.1182/blood.2018889493
- Primary Citation of Related Structures:
6NAN - PubMed Abstract:
Ixolaris is a potent tick salivary anticoagulant that binds coagulation factor Xa (FXa) and zymogen FX, with formation of a quaternary tissue factor (TF)/FVIIa/ FX(a)/Ixolaris inhibitory complex. Ixolaris blocks TF-induced coagulation and PAR2 signaling and prevents thrombosis, tumor growth, and immune activation. We present a high-resolution structure and dynamics of Ixolaris and describe the structural basis for recognition of FX. Ixolaris consists of 2 Kunitz domains (K1 and K2) in which K2 is strikingly dynamic and encompasses several residues involved in FX binding. This indicates that the backbone plasticity of K2 is critical for Ixolaris biological activity. Notably, a nuclear magnetic resonance-derived model reveals a mechanism for an electrostatically guided, high-affinity interaction between Ixolaris and FX heparin-binding (pro)exosite, resulting in an allosteric switch in the catalytic site. This is the first report revealing the structure-function relationship of an anticoagulant targeting a zymogen serving as a scaffold for TF inhibition.
- Department of Chemistry and Biochemistry, University of California, Santa Cruz, CA.
Organizational Affiliation: