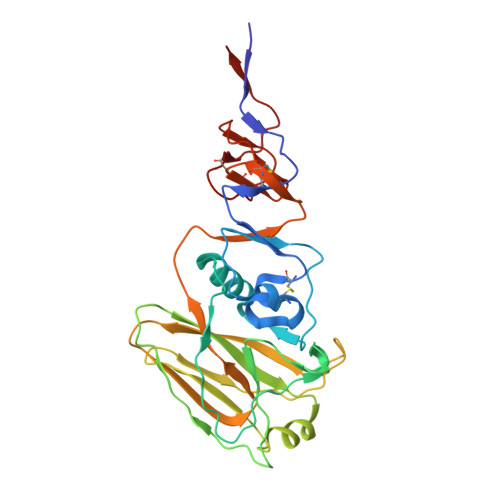
Influenza Antigen Engineering Focuses Immune Responses to a Subdominant but Broadly Protective Viral Epitope.
Bajic, G., Maron, M.J., Adachi, Y., Onodera, T., McCarthy, K.R., McGee, C.E., Sempowski, G.D., Takahashi, Y., Kelsoe, G., Kuraoka, M., Schmidt, A.G.(2019) Cell Host Microbe 25: 827
- PubMed: 31104946
- DOI: https://doi.org/10.1016/j.chom.2019.04.003
- Primary Citation of Related Structures:
6N5B, 6N5D, 6N5E - PubMed Abstract:
Viral glycoproteins are under constant immune surveillance by a host's adaptive immune responses. Antigenic variation including glycan introduction or removal is among the mechanisms viruses have evolved to escape host immunity. Understanding how glycosylation affects immunodominance on complex protein antigens may help decipher underlying B cell biology. To determine how B cell responses can be altered by such modifications, we engineered glycans onto the influenza virus hemagglutinin (HA) and characterized the molecular features of the elicited humoral immunity in mice. We found that glycan addition changed the initially diverse antibody repertoire into an epitope-focused, genetically restricted response. Structural analyses showed that one antibody gene family targeted a previously subdominant, occluded epitope at the head interface. Passive transfer of this antibody conferred Fc-dependent protection to influenza virus-challenged mice. These results have potential implications for next-generation viral vaccines aimed at directing B cell responses to preferred epitope(s).
- Laboratory of Molecular Medicine, Boston Children's Hospital, Harvard Medical School, Boston, MA 02115, USA.
Organizational Affiliation: