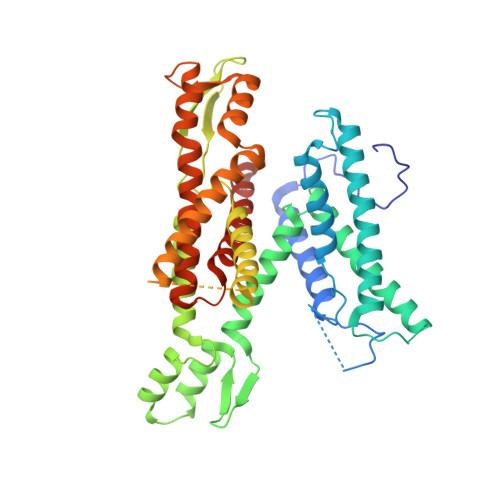
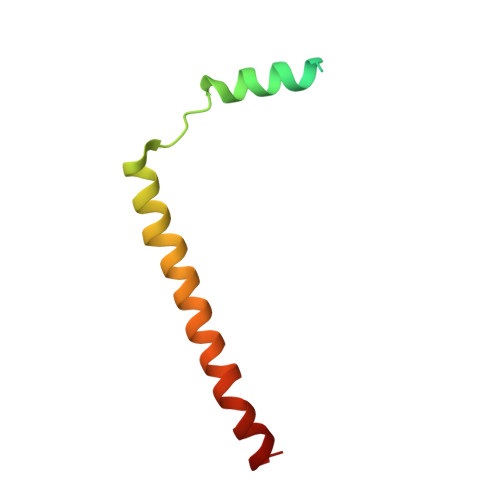
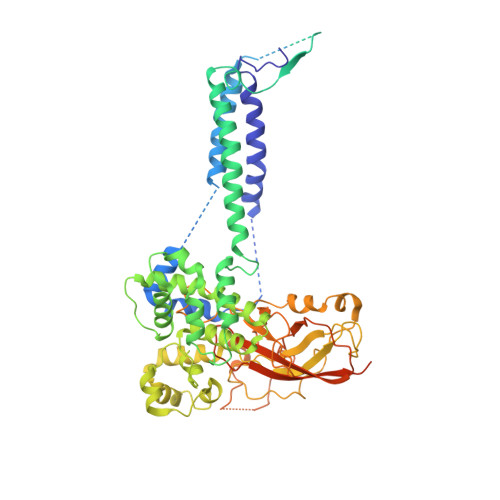
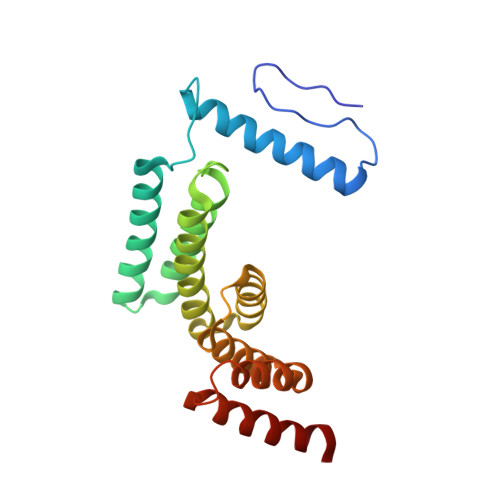
Structure of the posttranslational Sec protein-translocation channel complex from yeast.
Itskanov, S., Park, E.(2019) Science 363: 84-87
- PubMed: 30545845
- DOI: https://doi.org/10.1126/science.aav6740
- Primary Citation of Related Structures:
6N3Q - PubMed Abstract:
The Sec61 protein-conducting channel mediates transport of many proteins, such as secretory proteins, across the endoplasmic reticulum (ER) membrane during or after translation. Posttranslational transport is enabled by two additional membrane proteins associated with the channel, Sec63 and Sec62, but its mechanism is poorly understood. We determined a structure of the Sec complex (Sec61-Sec63-Sec71-Sec72) from Saccharomyces cerevisiae by cryo-electron microscopy (cryo-EM). The structure shows that Sec63 tightly associates with Sec61 through interactions in cytosolic, transmembrane, and ER-luminal domains, prying open Sec61's lateral gate and translocation pore and thus activating the channel for substrate engagement. Furthermore, Sec63 optimally positions binding sites for cytosolic and luminal chaperones in the complex to enable efficient polypeptide translocation. Our study provides mechanistic insights into eukaryotic posttranslational protein translocation.
- Biophysics Graduate Program, University of California, Berkeley, Berkeley, CA 94720, USA.
Organizational Affiliation: