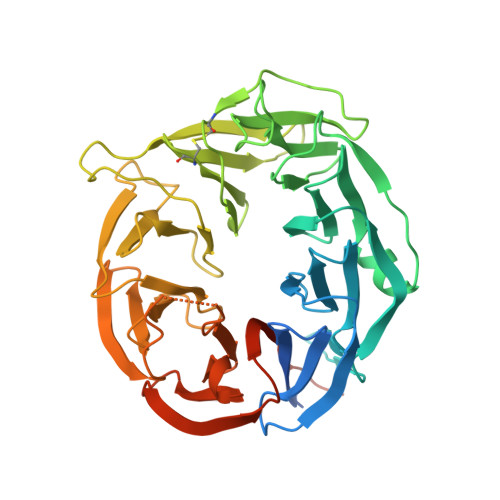
Crystal structures of AztD provide mechanistic insights into direct zinc transfer between proteins.
Neupane, D.P., Fullam, S.H., Chacon, K.N., Yukl, E.T.(2019) Commun Biol 2: 308-308
- PubMed: 31428696
- DOI: https://doi.org/10.1038/s42003-019-0542-z
- Primary Citation of Related Structures:
6CK1, 6CMK, 6N01 - PubMed Abstract:
Zinc acquisition from limited environments is critical for bacterial survival and pathogenesis. AztD has been identified as a periplasmic or cell surface zinc-binding protein in numerous bacterial species. In Paracoccus denitrificans , AztD can transfer zinc directly to AztC, the solute binding protein for a zinc-specific ATP-binding cassette transporter system, suggesting a role in zinc acquisition and homeostasis. Here, we present the first cry stal structures of AztD from P. denitrificans and tbe human pathogen Citrobacter koseri , revealing a beta-propeller fold and two high-affinity zinc-binding sites that are highly conserved among AztD homologs. These structures combined with transfer assays using WT and mutant proteins provide rare insight into the mechanism of direct zinc transfer from one protein to another. Given the importance of zinc import to bacterial pathogenesis, these insights may prove valuable to the development of zinc transfer inhibitors as antibiotics.
- 1Department of Chemistry and Biochemistry, New Mexico State University, Las Cruces, NM 88003 USA.
Organizational Affiliation: