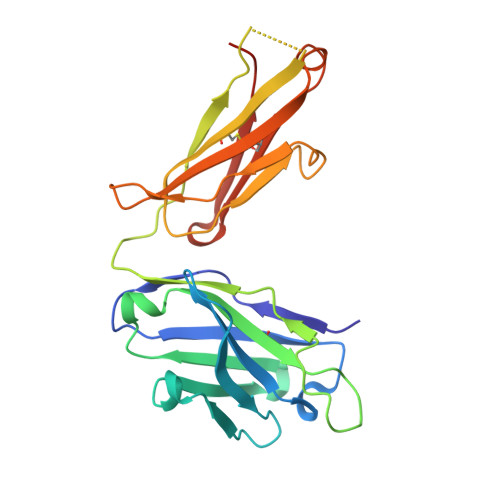
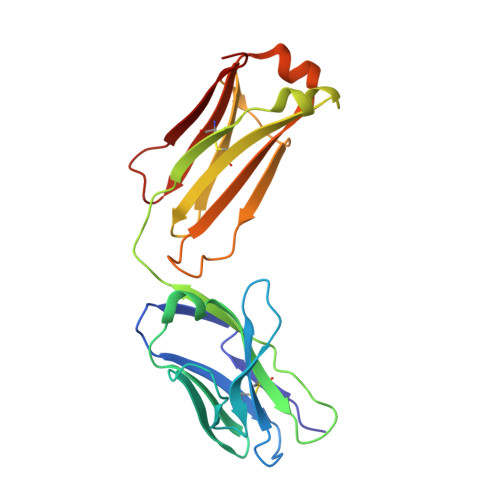
Boosting with AIDSVAX B/E Enhances Env Constant Region 1 and 2 Antibody-Dependent Cellular Cytotoxicity Breadth and Potency.
Easterhoff, D., Pollara, J., Luo, K., Tolbert, W.D., Young, B., Mielke, D., Jha, S., O'Connell, R.J., Vasan, S., Kim, J., Michael, N.L., Excler, J.L., Robb, M.L., Rerks-Ngarm, S., Kaewkungwal, J., Pitisuttithum, P., Nitayaphan, S., Sinangil, F., Tartaglia, J., Phogat, S., Kepler, T.B., Alam, S.M., Wiehe, K., Saunders, K.O., Montefiori, D.C., Tomaras, G.D., Moody, M.A., Pazgier, M., Haynes, B.F., Ferrari, G.(2020) J Virol 94
- PubMed: 31776278
- DOI: https://doi.org/10.1128/JVI.01120-19
- Primary Citation of Related Structures:
6MFJ, 6MFP - PubMed Abstract:
Induction of protective antibodies is a critical goal of HIV-1 vaccine development. One strategy is to induce nonneutralizing antibodies (NNAbs) that kill virus-infected cells, as these antibody specificities have been implicated in slowing HIV-1 disease progression and in protection. HIV-1 Env constant region 1 and 2 (C1C2) monoclonal antibodies (MAbs) frequently mediate potent antibody-dependent cellular cytotoxicity (ADCC), making them an important vaccine target. Here, we explore the effect of delayed and repetitive boosting of RV144 vaccine recipients with AIDSVAX B/E on the C1C2-specific MAb repertoire. It was found that boosting increased clonal lineage-specific ADCC breadth and potency. A ligand crystal structure of a vaccine-induced broad and potent ADCC-mediating C1C2-specific MAb showed that it bound a highly conserved Env gp120 epitope. Thus, boosting to affinity mature these types of IgG C1C2-specific antibody responses may be one method by which to make an improved HIV vaccine with higher efficacy than that seen in the RV144 trial. IMPORTANCE Over one million people become infected with HIV-1 each year, making the development of an efficacious HIV-1 vaccine an important unmet medical need. The RV144 human HIV-1 vaccine regimen is the only HIV-1 clinical trial to date to demonstrate vaccine efficacy. An area of focus has been on identifying ways by which to improve upon RV144 vaccine efficacy. The RV305 HIV-1 vaccine regimen was a follow-up boost of RV144 vaccine recipients that occurred 6 to 8 years after the conclusion of RV144. Our study focused on the effect of delayed boosting in humans on the vaccine-induced Env constant region 1 and 2 (C1C2)-specific antibody repertoire. It was found that boosting with an HIV-1 Env vaccine increased C1C2-specific antibody-dependent cellular cytotoxicity potency and breadth.
- Duke University, Durham, North Carolina, USA david.easterhoff@duke.edu guido.ferrari@duke.edu.
Organizational Affiliation: