Structural basis of self-assembly in the lipid-binding domain of mycobacterial polar growth factor Wag31
Chaudhuri, B.N., Choukate, K.(2020) IUCrJ 7: 767-776
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2020) IUCrJ 7: 767-776
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cell wall synthesis protein Wag31 | 74 | Mycobacterium tuberculosis H37Rv | Mutation(s): 0 Gene Names: wag31, ag84, Rv2145c, MTCY270.23 | 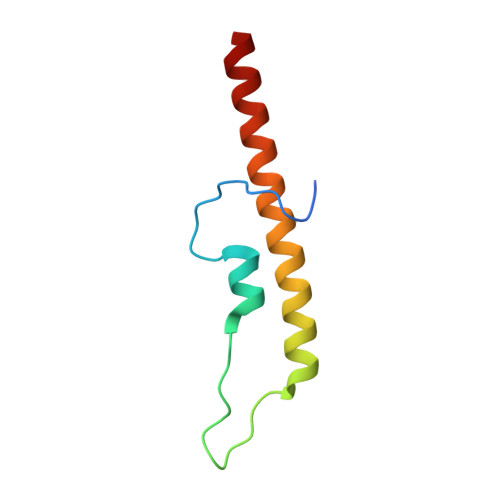 | |
UniProt | |||||
Find proteins for P9WMU1 (Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)) Explore P9WMU1 Go to UniProtKB: P9WMU1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P9WMU1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PGE Query on PGE | C [auth A] | TRIETHYLENE GLYCOL C6 H14 O4 ZIBGPFATKBEMQZ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 44.988 | α = 90 |
| b = 53.768 | β = 100.793 |
| c = 61.24 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| MOLREP | phasing |
| Aimless | data scaling |
| Coot | model building |
| XDS | data reduction |