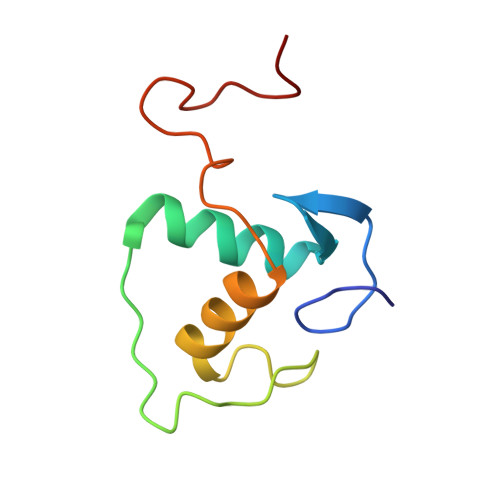
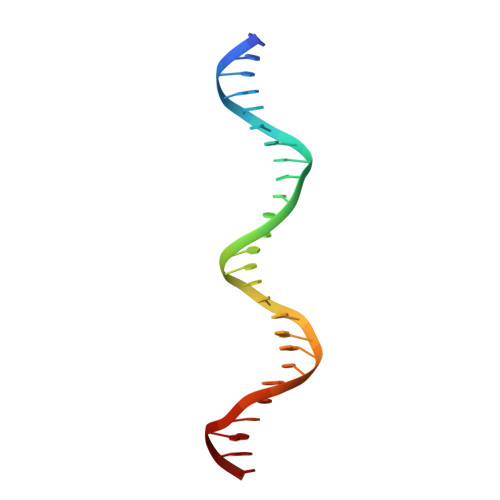
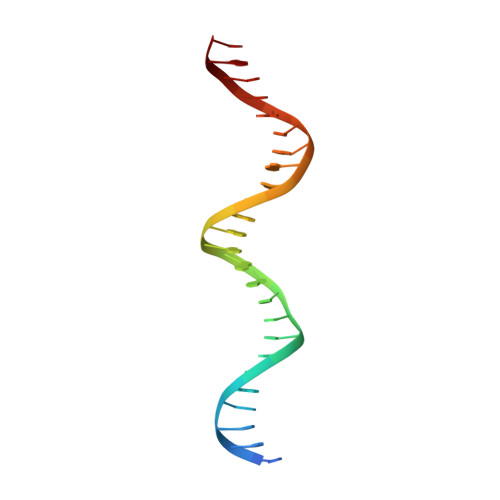
Structural basis of NR4A1 bound to the human pituitary proopiomelanocortin gene promoter.
Jiang, L., Wei, H., Yan, N., Dai, S., Li, J., Qu, L., Chen, X., Guo, M., Chen, Z., Chen, Y.(2020) Biochem Biophys Res Commun 523: 1-5
- PubMed: 31822342
- DOI: https://doi.org/10.1016/j.bbrc.2019.11.192
- Primary Citation of Related Structures:
6LC1 - PubMed Abstract:
The nuclear receptor NR4A subfamily (NR4A1/NGFI-B, NR4A2/Nurr1 and NR4A3/NOR-1) can recognize different classes of DNA response elements either as a monomer, homodimer, or heterodimer. In this study, we determined the structure of the NR4A1 DNA-binding domain (NR4A1-DBD) bound to natural Nur-responsive elements (NurREs) in the promoter region of the pituitary proopiomelanocortin (POMC) gene (NurRE POMC ) at 3.12 Å resolution. The NR4A1-DBD molecules bound independently to this element in our structure. The N-terminal helix H1 forms specific contacts with major groove, and C-terminal extension interact extensively with minor groove. Moreover our modelling structure of NR4A1 large fragment complexed with NurRE POMC indicated that ligand binding domain of NR4A might form dimer interactions to help recognize DNA. Overall, our analyses provide a molecular basis for DNA binding of NR4A proteins as a homodimer and novel insight into the molecular functions of NR4A modulation of gene expression.
- Department of Oncology, Laboratory of Structural Biology, NHC Key Laboratory of Cancer Proteomics, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China.
Organizational Affiliation: