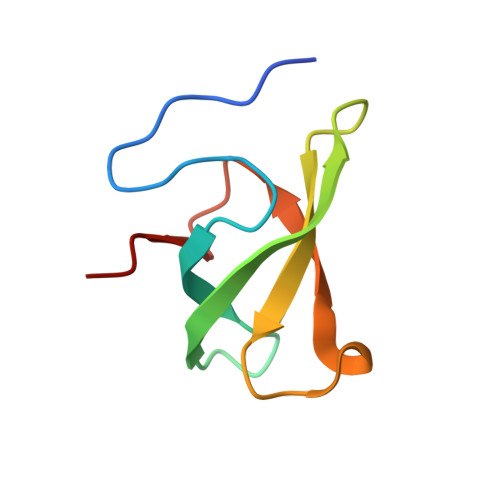
Conformational Selection in Ligand Recognition by the First Tudor Domain of PHF20L1.
Lv, M., Gao, J., Li, M., Ma, R., Li, F., Liu, Y., Liu, M., Zhang, J., Yao, X., Wu, J., Shi, Y., Tang, Y., Pan, Y., Zhang, Z., Ruan, K.(2020) J Phys Chem Lett 11: 7932-7938
- PubMed: 32885980
- DOI: https://doi.org/10.1021/acs.jpclett.0c02039
- Primary Citation of Related Structures:
6L0X, 6L10, 6L1C, 6L1F, 6L1I, 6L1P - PubMed Abstract:
The first Tudor domain (Tudor1) of PHF20L1 recognizes (non)histone methylation to play versatile roles. However, the underlying ligand-recognition mechanism remains unknown as a closed state revealed in the free-form structure. NMR relaxation dispersion and molecular dynamics simulations suggest a pre-existing low-population conformation with a remarkable rearrangement of aromatic cage residues of PHF20L1 Tudor1. Such an open-form conformation is utilized to recognize lysine 142 methylated DNMT1, a cosolvent, and an NMR fragment screening hit, as revealed by the complex crystal structures. Intriguingly, the ligand binding capacity was enhanced by mutation that tunes up the open-state population only. The recognition of DNMT1 by PHF20L1 was further validated in cancer cells. This conformational selection mechanism will enable the discovery of small molecule inhibitors against the seemingly "undruggable" PHF20L1 Tudor1.
- Ministry of Education Key Laboratory for Membrane-less Organelles & Cellular Dynamics, Hefei National Laboratory for Physical Sciences at the Microscale, the First Affiliated Hospital & School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230027, P.R. China.
Organizational Affiliation: