Mechanistic insights into the interactions of dynein regulator Ndel1 with neuronal ankyrins and implications in polarity maintenance.
Ye, J., Li, J., Ye, F., Zhang, Y., Zhang, M., Wang, C.(2020) Proc Natl Acad Sci U S A 117: 1207-1215
- PubMed: 31889000
- DOI: https://doi.org/10.1073/pnas.1916987117
- Primary Citation of Related Structures:
6KZJ - PubMed Abstract:
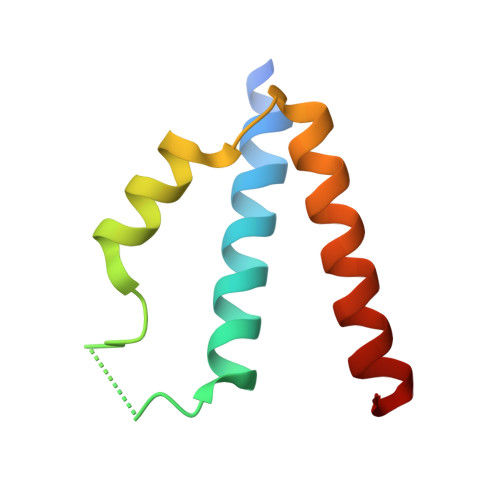
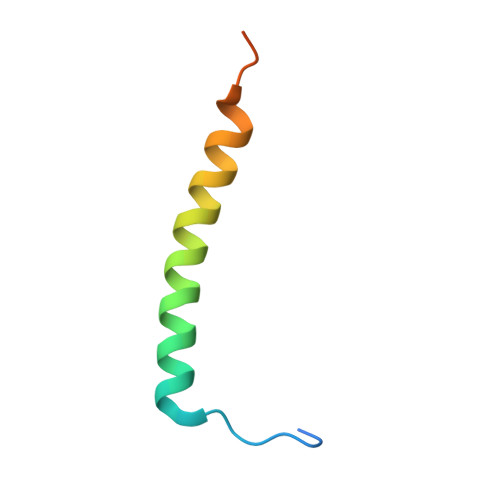
Ankyrin-G (AnkG), a highly enriched scaffold protein in the axon initial segment (AIS) of neurons, functions to maintain axonal polarity and the integrity of the AIS. At the AIS, AnkG regulates selective intracellular cargo trafficking between soma and axons via interaction with the dynein regulator protein Ndel1, but the molecular mechanism underlying this binding remains elusive. Here we report that Ndel1's C-terminal coiled-coil region (CT-CC) binds to giant neuron-specific insertion regions present in both AnkG and AnkB with 2:1 stoichiometry. The high-resolution crystal structure of AnkB in complex with Ndel1 CT-CC revealed the detailed molecular basis governing the AnkB/Ndel1 complex formation. Mechanistically, AnkB binds with Ndel1 by forming a stable 5-helix bundle dominated by hydrophobic interactions spread across 6 distinct interaction layers. Moreover, we found that AnkG is essential for Ndel1 accumulation at the AIS. Finally, we found that cargo sorting at the AIS can be disrupted by blocking the AnkG/Ndel1 complex formation using a peptide designed based on our structural data. Collectively, the atomic structure of the AnkB/Ndel1 complex together with studies of cargo sorting through the AIS establish the mechanistic basis for AnkG/Ndel1 complex formation and for the maintenance of axonal polarity. Our study will also be valuable for future studies of the interaction between AnkB and Ndel1 perhaps at distal axonal cargo transport.
- Ministry of Education Key Laboratory for Membrane-less Organelles & Cellular Dynamics, Hefei National Laboratory for Physical Sciences at the Microscale, School of Life Sciences, Division of Life Sciences and Medicine, University of Science and Technology of China, 230027 Hefei, P.R. China.
Organizational Affiliation: