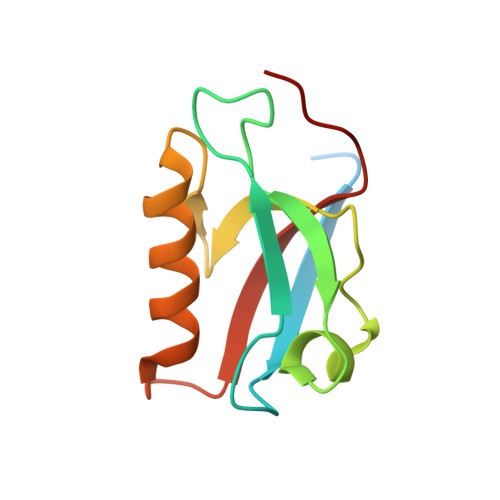
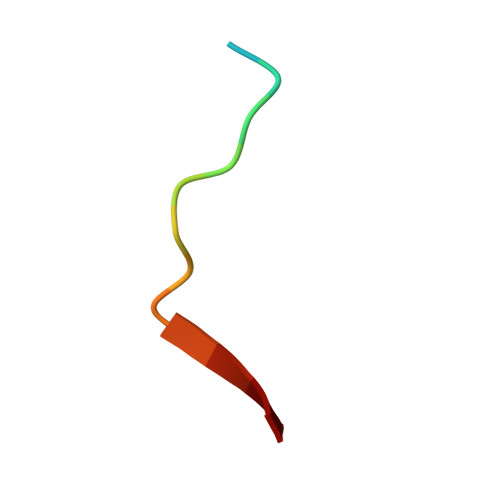
Phase separation-mediated condensation of Whirlin-Myo15-Eps8 stereocilia tip complex.
Lin, L., Shi, Y., Wang, M., Wang, C., Lu, Q., Zhu, J., Zhang, R.(2021) Cell Rep 34: 108770-108770
- PubMed: 33626355
- DOI: https://doi.org/10.1016/j.celrep.2021.108770
- Primary Citation of Related Structures:
6KZ1 - PubMed Abstract:
Stereocilia, the mechanosensory organelles on the apical surface of hair cells, are necessary to detect sound and carry out mechano-electrical transduction. An electron-dense matrix is located at the distal tips of stereocilia and plays crucial roles in the regulation of stereocilia morphology. Mutations of the components in this tip complex density (TCD) have been associated with profound deafness. However, the mechanism underlying the formation of the TCD is largely unknown. Here, we discover that the specific multivalent interactions among the Whirlin-myosin 15 (Myo15)-Eps8 complex lead to the formation of the TCD-like condensates through liquid-liquid phase separation. The reconstituted TCD-like condensates effectively promote actin bundling. A deafness-associated mutation of Myo15 interferes with the condensates formation and consequently impairs actin bundling. Therefore, our study not only suggests that the TCD in hair cell stereocilia may form via phase separation but it also provides important clues for the possible mechanism underlying hearing loss.
- State Key Laboratory of Molecular Biology, Shanghai Institute of Biochemistry and Cell Biology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China; Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders, Ministry of Education, Shanghai Jiao Tong University, Shanghai 200240, China.
Organizational Affiliation: