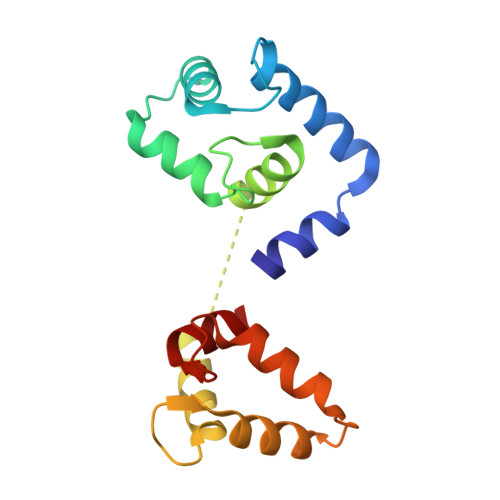
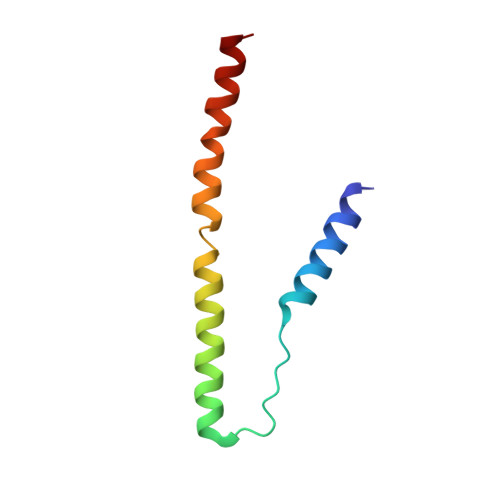
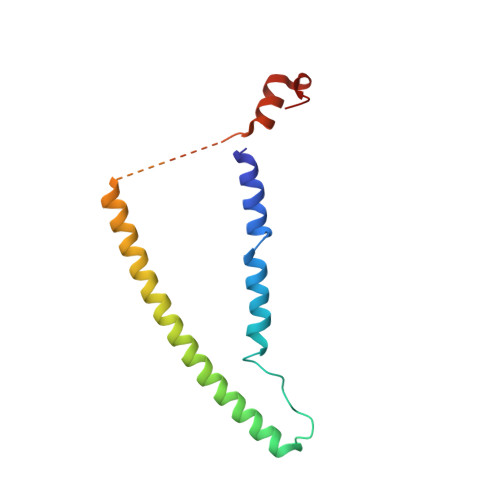
Cryo-EM structures of cardiac thin filaments reveal the 3D architecture of troponin.
Oda, T., Yanagisawa, H., Wakabayashi, T.(2020) J Struct Biol 209: 107450-107450
- PubMed: 31954841
- DOI: https://doi.org/10.1016/j.jsb.2020.107450
- Primary Citation of Related Structures:
6KLL, 6KLN, 6KLP, 6KLQ, 6KLT, 6KLU - PubMed Abstract:
Troponin is an essential component of striated muscle and it regulates the sliding of actomyosin system in a calcium-dependent manner. Despite its importance, the structure of troponin has been elusive due to its high structural heterogeneity. In this study, we analyzed the 3D structures of murine cardiac thin filaments using a cryo-electron microscope equipped with a Volta phase plate (VPP). Contrast enhancement by a VPP enabled us to reconstruct the entire repeat of the thin filament. We determined the orientation of troponin relative to F-actin and tropomyosin, and characterized the interactions between troponin and tropomyosin. This study provides a structural basis for understanding the molecular mechanism of actomyosin system.
- Department of Anatomy and Structural Biology, Graduate School of Medicine, University of Yamanashi, 1110 Shimokato, Chuo, Yamanashi 409-3898, Japan. Electronic address: toda@yamanashi.ac.jp.
Organizational Affiliation: