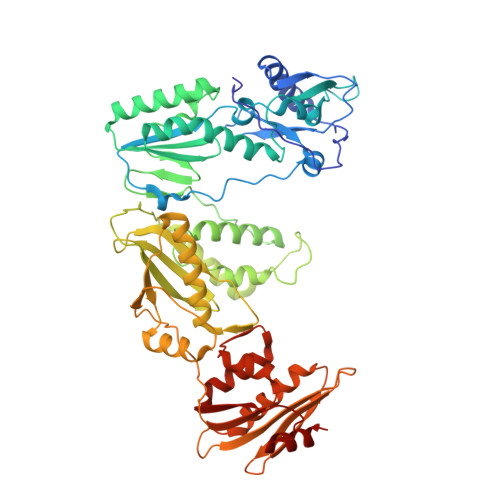
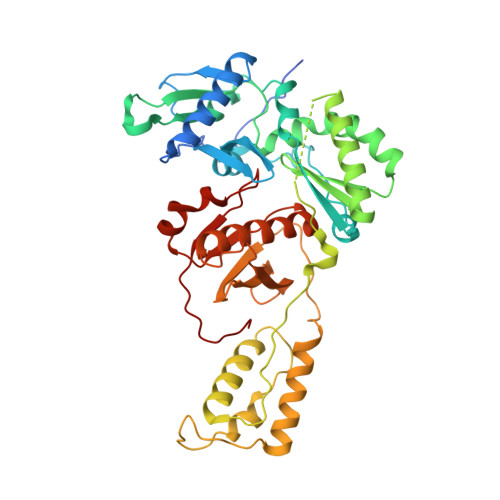
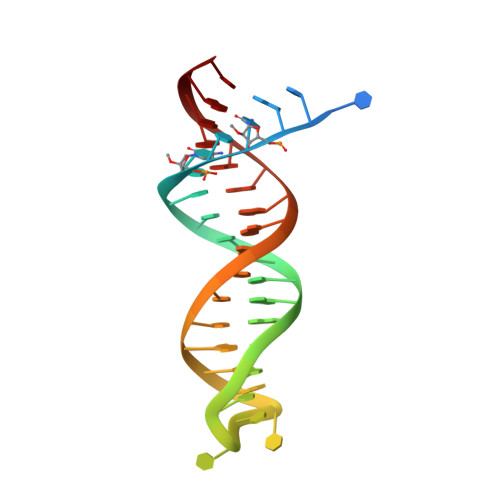
Structural features in common of HBV and HIV-1 resistance against chirally-distinct nucleoside analogues entecavir and lamivudine.
Yasutake, Y., Hattori, S.I., Tamura, N., Matsuda, K., Kohgo, S., Maeda, K., Mitsuya, H.(2020) Sci Rep 10: 3021-3021
- PubMed: 32080249
- DOI: https://doi.org/10.1038/s41598-020-59775-w
- Primary Citation of Related Structures:
6KDJ, 6KDK, 6KDM, 6KDN, 6KDO - PubMed Abstract:
Chronic hepatitis B virus (HBV) infection is a major public health problem that affects millions of people worldwide. Nucleoside analogue reverse transcriptase (RT) inhibitors, such as entecavir (ETV) and lamivudine (3TC), serve as crucial anti-HBV drugs. However, structural studies of HBV RT have been hampered due to its unexpectedly poor solubility. Here, we show that human immunodeficiency virus type-1 (HIV-1) with HBV-associated amino acid substitutions Y115F/F116Y/Q151M in its RT (HIV Y115F/F116Y/Q151M ) is highly susceptible to ETV and 3TC. Additionally, we experimentally simulated previously reported ETV/3TC resistance for HBV using HIV Y115F/F116Y/Q151M with F160M/M184V (L180M/M204V in HBV RT) substituted. We determined crystal structures for HIV-1 RT Y115F/F116Y/Q151M :DNA complexed with 3TC-triphosphate (3TC-TP)/ETV-triphosphate (ETV-TP)/dCTP/dGTP. These structures revealed an atypically tight binding conformation of 3TC-TP, where the Met184 side-chain is pushed away by the oxathiolane of 3TC-TP and exocyclic methylene of ETV-TP. Structural analysis of RT Y115F/F116Y/Q151M/F160M/M184V :DNA:3TC-TP also demonstrated that the loosely bound 3TC-TP is misaligned at the active site to prevent a steric clash with the side chain γ-methyl of Val184. These findings shed light on the common structural mechanism of HBV and HIV-1 resistance to 3TC and ETV and should aid in the design of new agents to overcome drug resistance to 3TC and ETV.
- Bioproduction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Sapporo, 062-8517, Japan. y-yasutake@aist.go.jp.
Organizational Affiliation: