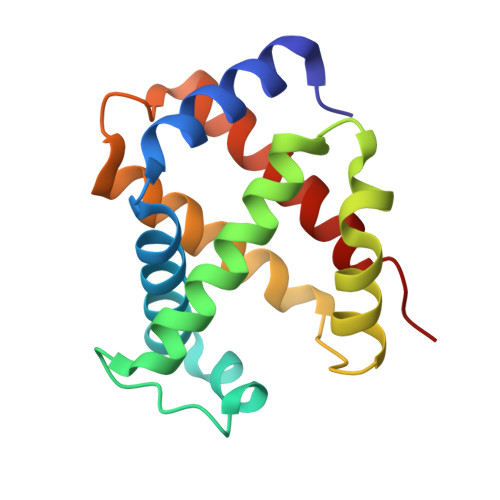
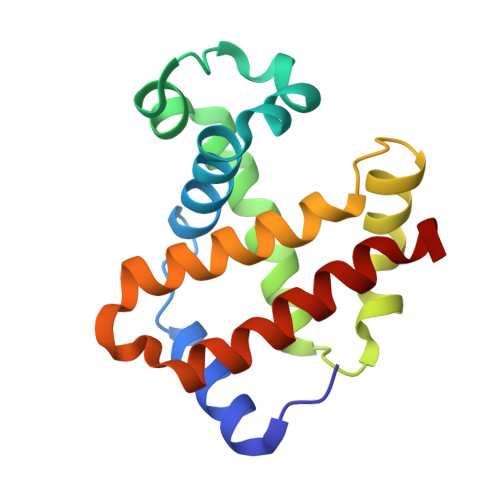
Direct observation of ligand migration within human hemoglobin at work.
Shibayama, N., Sato-Tomita, A., Ohki, M., Ichiyanagi, K., Park, S.Y.(2020) Proc Natl Acad Sci U S A 117: 4741-4748
- PubMed: 32071219
- DOI: https://doi.org/10.1073/pnas.1913663117
- Primary Citation of Related Structures:
6KA9, 6KAE, 6KAH, 6KAI, 6KAO, 6KAP, 6KAQ, 6KAR, 6KAS, 6KAT, 6KAU, 6KAV, 6L5V, 6L5W, 6L5X, 6L5Y, 6LCW, 6LCX - PubMed Abstract:
Hemoglobin is one of the best-characterized proteins with respect to structure and function, but the internal ligand diffusion pathways remain obscure and controversial. Here we captured the CO migration processes in the tense (T), relaxed (R), and second relaxed (R2) quaternary structures of human hemoglobin by crystallography using a high-repetition pulsed laser technique at cryogenic temperatures. We found that in each quaternary structure, the photodissociated CO molecules migrate along distinct pathways in the α and β subunits by hopping between the internal cavities with correlated side chain motions of large nonpolar residues, such as α14Trp(A12), α105Leu(G12), β15Trp(A12), and β71Phe(E15). We also observe electron density evidence for the distal histidine [α58/β63His(E7)] swing-out motion regardless of the quaternary structure, although less evident in α subunits than in β subunits, suggesting that some CO molecules have escaped directly through the E7 gate. Remarkably, in T-state Fe(II)-Ni(II) hybrid hemoglobins in which either the α or β subunits contain Ni(II) heme that cannot bind CO, the photodissociated CO molecules not only dock at the cavities in the original Fe(II) subunit, but also escape from the protein matrix and enter the cavities in the adjacent Ni(II) subunit even at 95 K, demonstrating the high gas permeability and porosity of the hemoglobin molecule. Our results provide a comprehensive picture of ligand movements in hemoglobin and highlight the relevance of cavities, nonpolar residues, and distal histidines in facilitating the ligand migration.
- Division of Biophysics, Department of Physiology, Jichi Medical University, Tochigi, 329-0498 Shimotsuke, Japan; shibayam@jichi.ac.jp.
Organizational Affiliation: