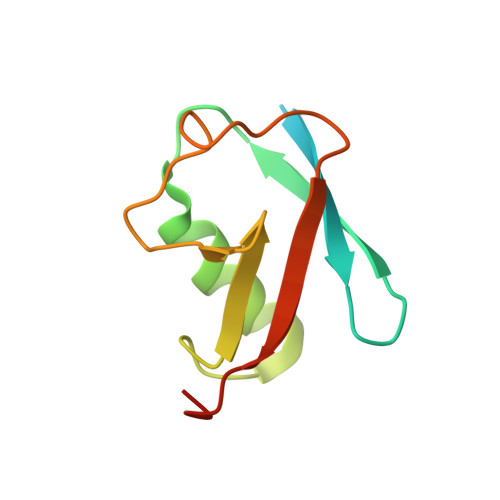
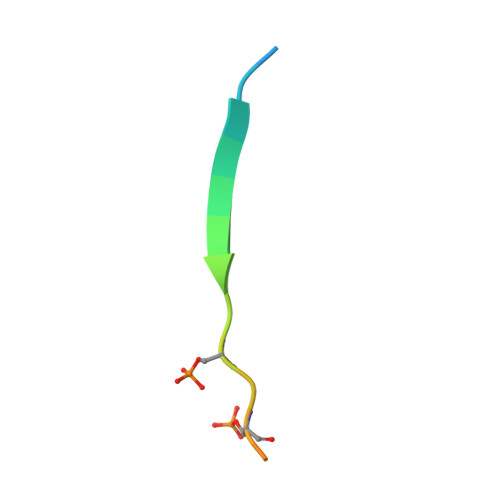
The Viral SUMO-Targeted Ubiquitin Ligase ICP0 is Phosphorylated and Activated by Host Kinase Chk2.
Hembram, D.S.S., Negi, H., Biswas, P., Tripathi, V., Bhushan, L., Shet, D., Kumar, V., Das, R.(2020) J Mol Biology 432: 1952-1977
- PubMed: 32001251
- DOI: https://doi.org/10.1016/j.jmb.2020.01.021
- Primary Citation of Related Structures:
6JXU, 6JXV, 6JXW, 6JXX - PubMed Abstract:
When the herpes simplex virus (HSV) genome enters the nucleus for replication and transcription, phase-segregated nuclear protein bodies called Promyelocytic leukemia protein nuclear bodies (PML NBs) colocalize with the genome and repress it. HSV encodes a small ubiquitin-like modifier (SUMO)-targeted ubiquitin ligase (STUbL) infected cell polypeptide 0 (ICP0) that degrades PML NBs to alleviate the repression. The molecular details of the mechanism used by ICP0 to target PML NBs are unclear. Here, we identify a bona fide SUMO-interacting motif in ICP0 (SIM-like sequence [SLS] 4) that is essential and sufficient to target SUMOylated proteins in PML NBs such as the PML and Sp100. We shown that phosphorylation of SLS4 creates new salt bridges between SUMO and SLS4, increases the SUMO/SLS4 affinity, and switches ICP0 into a potent STUbL. HSV activates the Ataxia-telangiectasia-mutated kinase-Checkpoint kinase 2 (ATM-Chk2) pathway to regulate the cell cycle of the host. We report that the activated Chk2 also phosphorylates ICP0 at SLS4 and enhances its STUbL activity. Our results uncover that a viral STUbL counters antiviral response by exploiting an unprecedented cross-talk of three post-translational modifications: ubiquitination, SUMOylation, and phosphorylation.
- National Center for Biological Sciences, TIFR, Bangalore, India.
Organizational Affiliation: