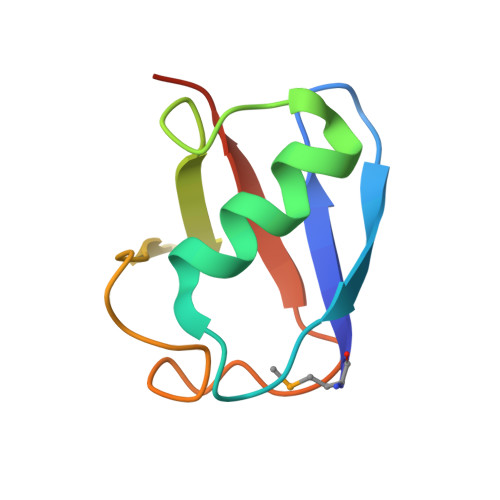
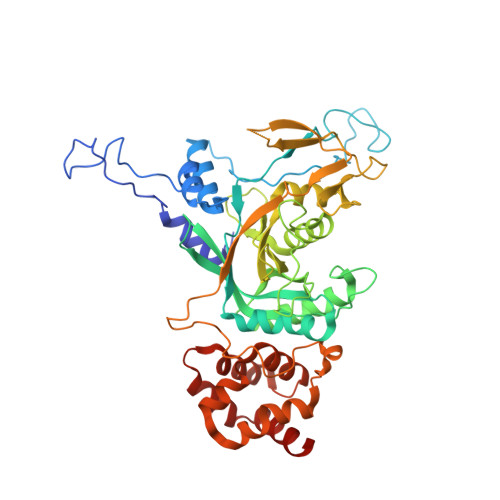
Structural insights into ubiquitin recognition and Ufd1 interaction of Npl4.
Sato, Y., Tsuchiya, H., Yamagata, A., Okatsu, K., Tanaka, K., Saeki, Y., Fukai, S.(2019) Nat Commun 10: 5708-5708
- PubMed: 31836717
- DOI: https://doi.org/10.1038/s41467-019-13697-y
- Primary Citation of Related Structures:
6JWH, 6JWI, 6JWJ - PubMed Abstract:
Npl4 is likely to be the most upstream factor recognizing Lys48-linked polyubiquitylated substrates in the proteasomal degradation pathway in yeast. Along with Ufd1, Npl4 forms a heterodimer (UN), and functions as a cofactor for the Cdc48 ATPase. Here, we report the crystal structures of yeast Npl4 in complex with Lys48-linked diubiquitin and with the Npl4-binding motif of Ufd1. The distal and proximal ubiquitin moieties of Lys48-linked diubiquitin primarily interact with the C-terminal helix and N-terminal loop of the Npl4 C-terminal domain (CTD), respectively. Mutational analysis suggests that the CTD contributes to linkage selectivity and initial binding of ubiquitin chains. Ufd1 occupies a hydrophobic groove of the Mpr1/Pad1 N-terminal (MPN) domain of Npl4, which corresponds to the catalytic groove of the MPN domain of JAB1/MPN/Mov34 metalloenzyme (JAMM)-family deubiquitylating enzyme. This study provides important structural insights into the polyubiquitin chain recognition by the Cdc48-UN complex and its assembly.
- Institute for Quantitative Biosciences, The University of Tokyo, Tokyo, 113-0032, Japan.
Organizational Affiliation: