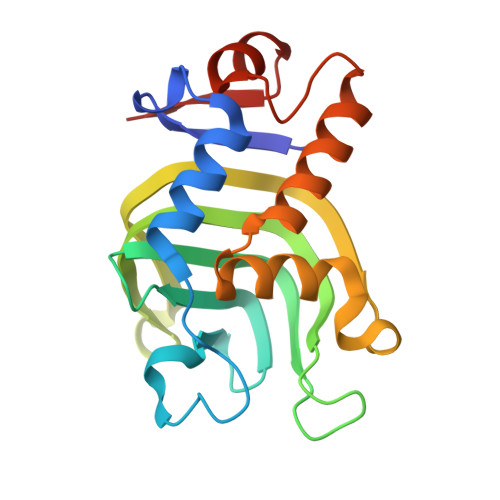
Highly malleable haem-binding site of the haemoprotein HasA permits stable accommodation of bulky tetraphenylporphycenes.
Sakakibara, E., Shisaka, Y., Onoda, H., Koga, D., Xu, N., Ono, T., Hisaeda, Y., Sugimoto, H., Shiro, Y., Watanabe, Y., Shoji, O.(2019) RSC Adv 9: 18697-18702