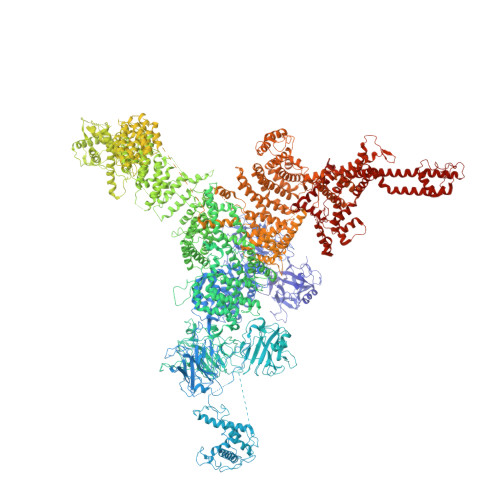
Molecular basis for allosteric regulation of the type 2 ryanodine receptor channel gating by key modulators.
Chi, X., Gong, D., Ren, K., Zhou, G., Huang, G., Lei, J., Zhou, Q., Yan, N.(2019) Proc Natl Acad Sci U S A 116: 25575-25582
- PubMed: 31792195
- DOI: https://doi.org/10.1073/pnas.1914451116
- Primary Citation of Related Structures:
6JG3, 6JGZ, 6JH6, 6JHN - PubMed Abstract:
The type 2 ryanodine receptor (RyR2) is responsible for releasing Ca 2+ from the sarcoplasmic reticulum of cardiomyocytes, subsequently leading to muscle contraction. Here, we report 4 cryo-electron microscopy (cryo-EM) structures of porcine RyR2 bound to distinct modulators that, together with our published structures, provide mechanistic insight into RyR2 regulation. Ca 2+ alone induces a contraction of the central domain that facilitates the dilation of the S6 bundle but is insufficient to open the pore. The small-molecule agonist PCB95 helps Ca 2+ to overcome the barrier for opening. FKBP12.6 induces a relaxation of the central domain that decouples it from the S6 bundle, stabilizing RyR2 in a closed state even in the presence of Ca 2+ and PCB95. Although the channel is open when PCB95 is replaced by caffeine and adenosine 5'-triphosphate (ATP), neither of the modulators alone can sufficiently counter the antagonistic effect to open the channel. Our study marks an important step toward mechanistic understanding of the sophisticated regulation of this key channel whose aberrant activity engenders life-threatening cardiac disorders.
- Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, Hangzhou 310024, China.
Organizational Affiliation: