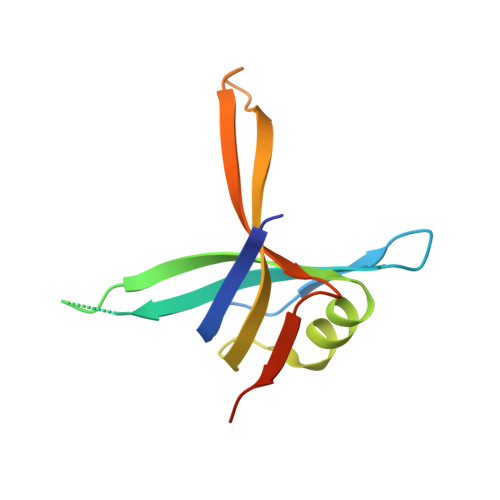
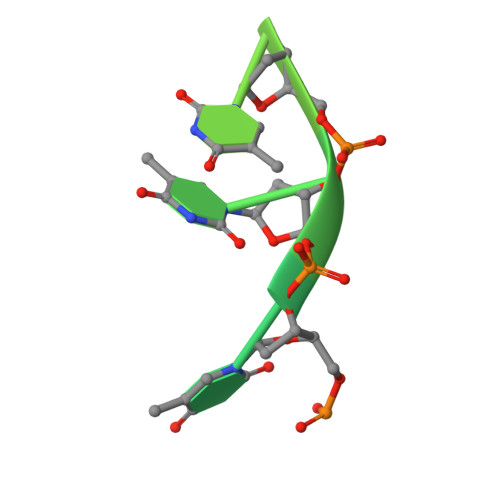
Complexed crystal structure of SSB reveals a novel single-stranded DNA binding mode (SSB)3:1: Phe60 is not crucial for defining binding paths.
Huang, Y.H., Lin, E.S., Huang, C.Y.(2019) Biochem Biophys Res Commun 520: 353-358
- PubMed: 31604524
- DOI: https://doi.org/10.1016/j.bbrc.2019.10.036
- Primary Citation of Related Structures:
6JDG - PubMed Abstract:
Single-stranded DNA-binding protein (SSB) is essential to cells as it participates in DNA metabolic processes, such as DNA replication, repair, and recombination. Escherichia coli SSB (EcSSB) tetramer cooperatively binds and wraps ssDNA in two major binding modes. In this study, we report the complex crystal structure of Pseudomonas aeruginosa SSB (PaSSB) with ssDNA dT20 at 2.39 Å resolution (PDB entry 6JDG) that revealed a new binding mode, namely, (SSB) 3:1 . In the (SSB) 65 mode revealed by the EcSSB-dC35 complex structure, all four subunits fully participate in the binding to ssDNA. However, only three subunits in the PaSSB tetramer can participate in wrapping ssDNA in the (SSB) 3:1 mode. The bound ssDNA in the PaSSB-ssDNA complex adopts an Ω-shaped conformation rather than a χ-shaped conformation in the (SSB) 65 mode possibly due to the disability of Phe60. Phe60 is known to play a critical role in defining DNA-binding paths and promoting the wrapping of ssDNA around SSB tetramers. However, it is not important in the (SSB) 3:1 mode. The ssDNA binding path revealed by our structural evidence suggests that ssDNA occupies half of the binding sites of the two subunits and slightly comes into contact with the ssDNA binding sites of the third subunit. Accordingly, we propose and sketch a possible wrapping mechanism of SSB via this novel ssDNA-binding mode, (SSB) 3:1 .
- School of Biomedical Sciences, Chung Shan Medical University, No.110, Sec.1, Chien-Kuo N. Rd., Taichung City, Taiwan.
Organizational Affiliation: