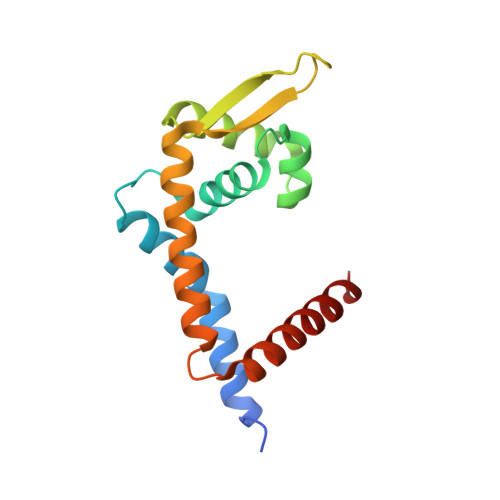
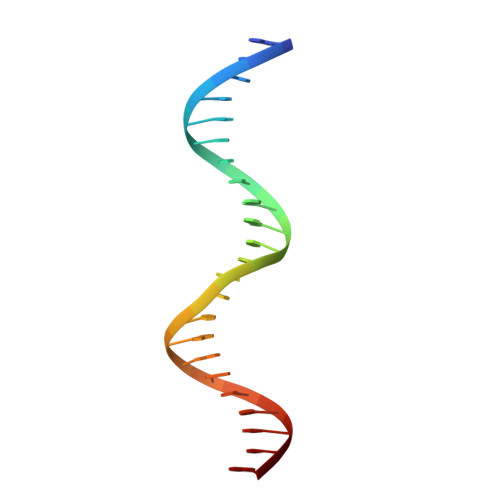
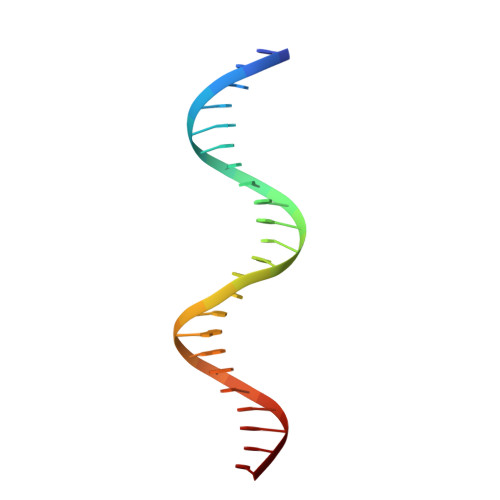
Structural insights into repression of the Pneumococcal fatty acid synthesis pathway by repressor FabT and co-repressor acyl-ACP.
Zuo, G., Chen, Z.P., Jiang, Y.L., Zhu, Z., Ding, C., Zhang, Z., Chen, Y., Zhou, C.Z., Li, Q.(2019) FEBS Lett 593: 2730-2741
- PubMed: 31291684
- DOI: https://doi.org/10.1002/1873-3468.13534
- Primary Citation of Related Structures:
6JBX - PubMed Abstract:
The Streptococcus pneumoniae fatty acid synthesis (FAS) pathway is globally controlled at the transcriptional level by the repressor FabT and its co-repressor acyl carrier protein (acyl-ACP), the intermediate of phospholipid synthesis. Here, we report the crystal structure of FabT complexed with a 23-bp dsDNA, which indicates that FabT is a weak repressor with low DNA-binding affinity in the absence of acyl-ACP. Modification of ACP with a long-chain fatty acid is necessary for the formation of a stable complex with FabT, mimicked in vitro by cross-linking, which significantly elevates the DNA-binding affinity of FabT. Altogether, we propose a putative working model of gene repression under the double control of FabT and acyl-ACP, elucidating a distinct repression network for Pneumococcus to precisely coordinate FAS.
- Hefei National Laboratory for Physical Sciences at the Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, China.
Organizational Affiliation: