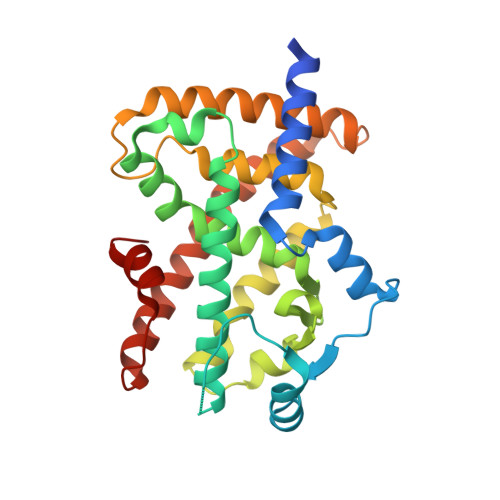
Structure-Activity Relationship Studies of 3- or 4-Pyridine Derivatives of DS-6930.
Shinozuka, T., Tsukada, T., Fujii, K., Tokumaru, E., Matsui, Y., Wakimoto, S., Ogata, T., Araki, K., Sawamura, R., Watanabe, N., Mori, M., Tanaka, J.(2019) ACS Med Chem Lett 10: 358-362
- PubMed: 30891140
- DOI: https://doi.org/10.1021/acsmedchemlett.8b00645
- Primary Citation of Related Structures:
6IZM, 6IZN - PubMed Abstract:
Derivatization efforts were continued to discover backups for a potent selective PPARγ modulator, DS-6930. In this Letter, the replacement of 2-pyridine ring in DS-6930 with 3- or 4-pyridyl group is reported. As the introduction of substituents on the pyridine ring did not provide potent partial agonists, modifications of benzimidazole ring were explored to discover potent intermediate agonists. 4'-Alkoxy substituted benzimidazoles failed to show potent efficacy in vivo, whereas 7'-fluoro benzimidazole 3g (DS19161384) was found to result in robust plasma glucose reductions with excellent DMPK profiles.
- R&D Division, Daiichi Sankyo Co., Ltd., 1-2-58 Hiromachi, Shinagawa-ku, Tokyo 140-8710, Japan.
Organizational Affiliation: