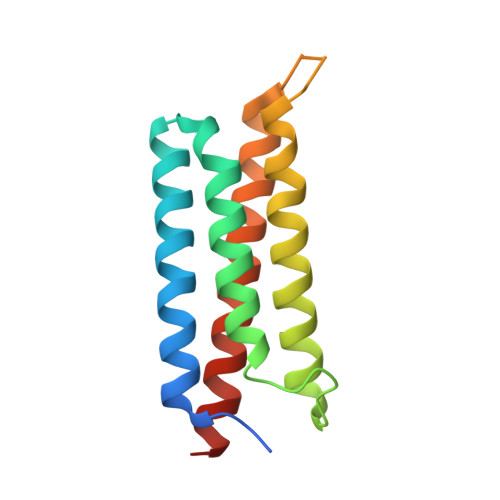
Structural basis of the target-binding mode of the G protein-coupled receptor kinase-interacting protein in the regulation of focal adhesion dynamics.
Liang, M., Xie, X., Pan, J., Jin, G., Yu, C., Wei, Z.(2019) J Biological Chem 294: 5827-5839
- PubMed: 30737283
- DOI: https://doi.org/10.1074/jbc.RA118.006915
- Primary Citation of Related Structures:
6IUH, 6IUI - PubMed Abstract:
Focal adhesions (FAs) are specialized sites where intracellular cytoskeleton elements connect to the extracellular matrix and thereby control cell motility. FA assembly depends on various scaffold proteins, including the G protein-coupled receptor kinase-interacting protein 1 (GIT1), paxillin, and liprin-α. Although liprin-α and paxillin are known to competitively interact with GIT1, the molecular basis governing these interactions remains elusive. To uncover the underlying mechanisms of how GIT1 is involved in FA assembly by alternatively binding to liprin-α and paxillin, here we solved the crystal structures of GIT1 in complex with liprin-α and paxillin at 1.8 and 2.6 Å resolutions, respectively. These structures revealed that the paxillin-binding domain (PBD) of GIT1 employs distinct binding modes to recognize a single α-helix of liprin-α and the LD4 motif of paxillin. Structure-based design of protein variants produced two binding-deficient GIT1 variants; specifically, these variants lost the ability to interact with liprin-α only or with both liprin-α and paxillin. Expressing the GIT1 variants in COS7 cells, we discovered that the two PBD-meditated interactions play different roles in either recruiting GIT1 to FA or facilitating FA assembly. Additionally, we demonstrate that, unlike for the known binding mode of the FAT domain to LD motifs, the PBD of GIT1 uses different surface patches to achieve high selectivity in LD motif recognition. In summary, our results have uncovered the mechanisms by which GIT1's PBD recognizes cognate paxillin and liprin-α structures, information we anticipate will be useful for future investigations of GIT1-protein interactions in cells.
- From the Department of Biology, Southern University of Science and Technology, Shenzhen 518055, China.
Organizational Affiliation: