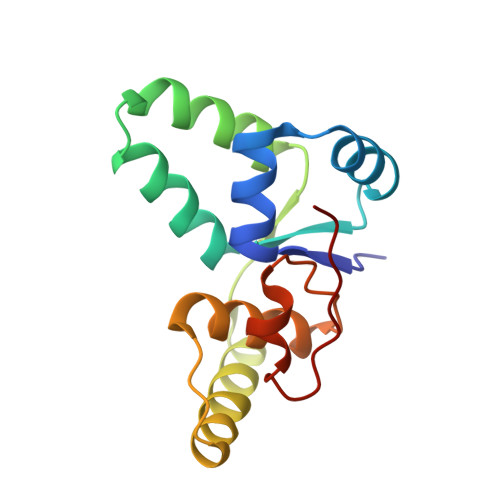
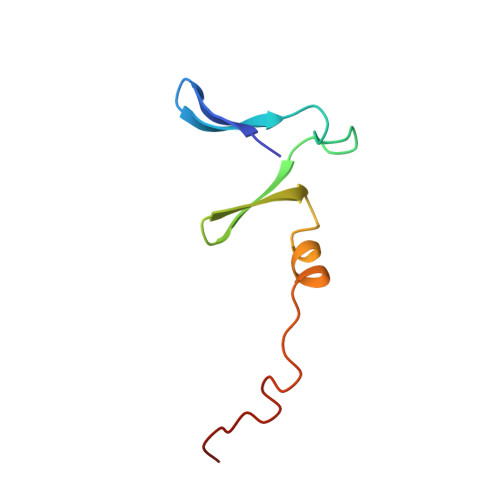
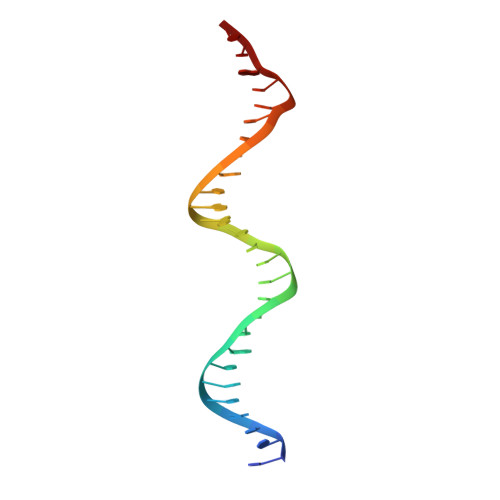
Crystal structure of proteolyzed VapBC and DNA-bound VapBC from Salmonella enterica Typhimurium LT2 and VapC as a putative Ca2+-dependent ribonuclease.
Park, D., Yoon, H.J., Lee, K.Y., Park, S.J., Cheon, S.H., Lee, H.H., Lee, S.J., Lee, B.J.(2020) FASEB J 34: 3051-3068
- PubMed: 31908032
- DOI: https://doi.org/10.1096/fj.201901989R
- Primary Citation of Related Structures:
6IFC, 6IFM - PubMed Abstract:
Bacterial toxin-antitoxin (TA) system has gained attention for its essential roles in cellular maintenance and survival under harsh environmental conditions such as nutrient deficiency and antibiotic treatment. There are at least 14 TA systems in Salmonella enterica serovar Typhimurium LT2, a pathogenic bacterium, and none of the structures of these TA systems have been determined. We determined the crystal structure of the VapBC TA complex from S. Typhimurium LT2 in proteolyzed and DNA-bound forms at 2.0 Å and 2.8 Å resolution, respectively. The VapC toxin possesses a pilT N-terminal domain (PIN-domain) that shows ribonuclease activity, and the VapB antitoxin has an AbrB-type DNA binding domain. In addition, the structure revealed details of interaction mode between VapBC and the cognate promoter DNA, including the inhibition of VapC by VapB and linear conformation of bound DNA in the VapBC complex. The complexation of VapBC with the linear DNA is not consistent with known structures of VapBC homologs in complex with bent DNA. We also identified VapC from S. Typhimurium LT2 as a putative Ca 2+ -dependent ribonuclease, which differs from previous data showing that VapC homologs have Mg 2+ or Mn 2+ -dependent ribonuclease activities. The present studies could provide structural understanding of the physiology of VapBC systems and foundation for the development of new antibiotic drugs against Salmonella infection.
- The Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul, Republic of Korea.
Organizational Affiliation: