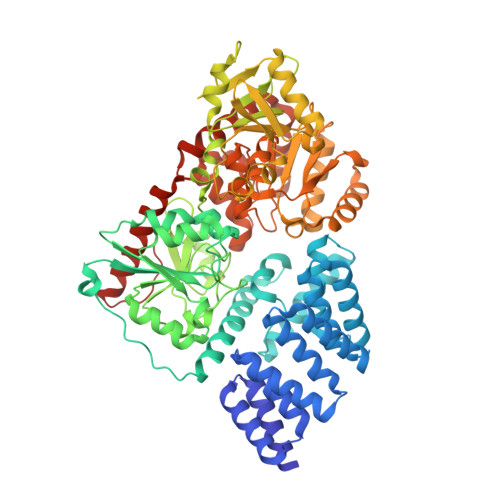
Catalytic deficiency of O-GlcNAc transferase leads to X-linked intellectual disability.
Pravata, V.M., Muha, V., Gundogdu, M., Ferenbach, A.T., Kakade, P.S., Vandadi, V., Wilmes, A.C., Borodkin, V.S., Joss, S., Stavridis, M.P., van Aalten, D.M.F.(2019) Proc Natl Acad Sci U S A 116: 14961-14970
- PubMed: 31296563
- DOI: https://doi.org/10.1073/pnas.1900065116
- Primary Citation of Related Structures:
6IBO - PubMed Abstract:
O-GlcNAc transferase (OGT) is an X-linked gene product that is essential for normal development of the vertebrate embryo. It catalyses the O-GlcNAc posttranslational modification of nucleocytoplasmic proteins and proteolytic maturation of the transcriptional coregulator Host cell factor 1 (HCF1). Recent studies have suggested that conservative missense mutations distal to the OGT catalytic domain lead to X-linked intellectual disability in boys, but it is not clear if this is through changes in the O-GlcNAc proteome, loss of protein-protein interactions, or misprocessing of HCF1. Here, we report an OGT catalytic domain missense mutation in monozygotic female twins (c. X:70779215 T > A, p. N567K) with intellectual disability that allows dissection of these effects. The patients show limited IQ with developmental delay and skewed X-inactivation. Molecular analyses revealed decreased OGT stability and disruption of the substrate binding site, resulting in loss of catalytic activity. Editing this mutation into the Drosophila genome results in global changes in the O-GlcNAc proteome, while in mouse embryonic stem cells it leads to loss of O-GlcNAcase and delayed differentiation down the neuronal lineage. These data imply that catalytic deficiency of OGT could contribute to X-linked intellectual disability.
- Division of Gene Regulation and Expression, School of Life Sciences, University of Dundee, DD1 5EH Dundee, United Kingdom.
Organizational Affiliation: