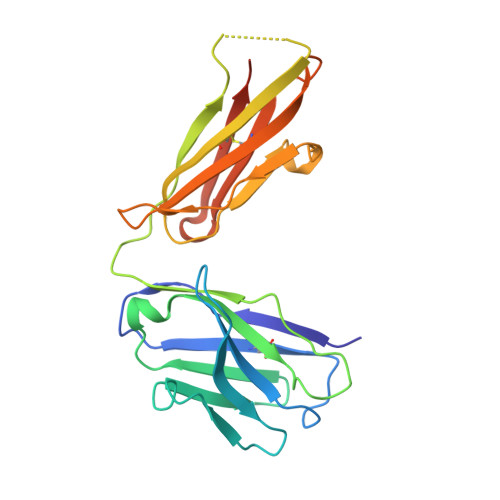
A Protective Monoclonal Antibody Targets a Site of Vulnerability on the Surface of Rift Valley Fever Virus.
Allen, E.R., Krumm, S.A., Raghwani, J., Halldorsson, S., Elliott, A., Graham, V.A., Koudriakova, E., Harlos, K., Wright, D., Warimwe, G.M., Brennan, B., Huiskonen, J.T., Dowall, S.D., Elliott, R.M., Pybus, O.G., Burton, D.R., Hewson, R., Doores, K.J., Bowden, T.A.(2018) Cell Rep 25: 3750-3758.e4
- PubMed: 30590046
- DOI: https://doi.org/10.1016/j.celrep.2018.12.001
- Primary Citation of Related Structures:
6I9I - PubMed Abstract:
The Gn subcomponent of the Gn-Gc assembly that envelopes the human and animal pathogen, Rift Valley fever virus (RVFV), is a primary target of the neutralizing antibody response. To better understand the molecular basis for immune recognition, we raised a class of neutralizing monoclonal antibodies (nAbs) against RVFV Gn, which exhibited protective efficacy in a mouse infection model. Structural characterization revealed that these nAbs were directed to the membrane-distal domain of RVFV Gn and likely prevented virus entry into a host cell by blocking fusogenic rearrangements of the Gn-Gc lattice. Genome sequence analysis confirmed that this region of the RVFV Gn-Gc assembly was under selective pressure and constituted a site of vulnerability on the virion surface. These data provide a blueprint for the rational design of immunotherapeutics and vaccines capable of preventing RVFV infection and a model for understanding Ab-mediated neutralization of bunyaviruses more generally.
- Division of Structural Biology, Wellcome Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK.
Organizational Affiliation: