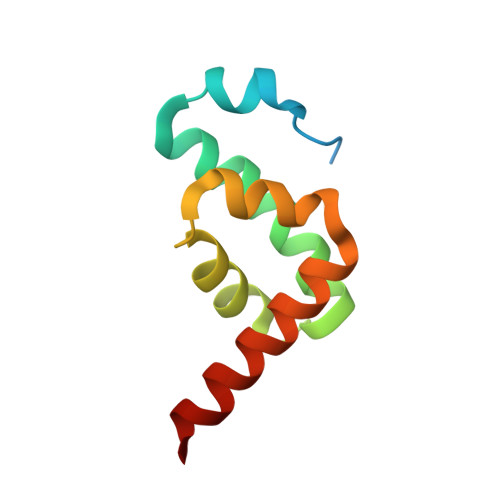
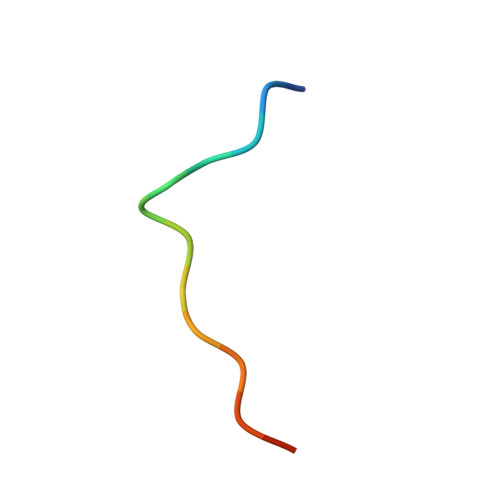
The Leishmania PABP1-eIF4E4 interface: a novel 5'-3' interaction architecture for trans-spliced mRNAs.
Dos Santos Rodrigues, F.H., Firczuk, H., Breeze, A.L., Cameron, A.D., Walko, M., Wilson, A.J., Zanchin, N.I.T., McCarthy, J.E.G.(2019) Nucleic Acids Res 47: 1493-1504
- PubMed: 30476241
- DOI: https://doi.org/10.1093/nar/gky1187
- Primary Citation of Related Structures:
6H7A, 6H7B - PubMed Abstract:
Trans-splicing of trypanosomatid polycistronic transcripts produces polyadenylated monocistronic mRNAs modified to form the 5' cap4 structure (m7Gpppm36,6,2'Apm2'Apm2'Cpm23,2'U). NMR and X-ray crystallography reveal that Leishmania has a unique type of N-terminally-extended cap-binding protein (eIF4E4) that binds via a PAM2 motif to PABP1. This relies on the interactions of a combination of polar and charged amino acid side-chains together with multiple hydrophobic interactions, and underpins a novel architecture in the Leishmania cap4-binding translation factor complex. Measurements using microscale thermophoresis, fluorescence anisotropy and surface plasmon resonance characterize the key interactions driving assembly of the Leishmania translation initiation complex. We demonstrate that this complex can accommodate Leishmania eIF4G3 which, unlike the standard eukaryotic initiation complex paradigm, binds tightly to eIF4E4, but not to PABP1. Thus, in Leishmania, the chain of interactions 5'cap4-eIF4E4-PABP1-poly(A) bridges the mRNA 5' and 3' ends. Exceptionally, therefore, by binding tightly to two protein ligands and to the mRNA 5' cap4 structure, the trypanosomatid N-terminally extended form of eIF4E acts as the core molecular scaffold for the mRNA-cap-binding complex. Finally, the eIF4E4 N-terminal extension is an intrinsically disordered region that transitions to a partly folded form upon binding to PABP1, whereby this interaction is not modulated by poly(A) binding to PABP1.
- Warwick Integrative Synthetic Biology Centre (WISB) and School of Life Sciences, University of Warwick, Gibbet Hill, Coventry CV4 7AL, UK.
Organizational Affiliation: