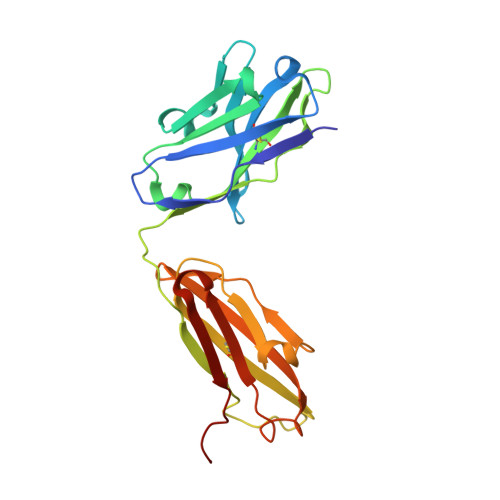
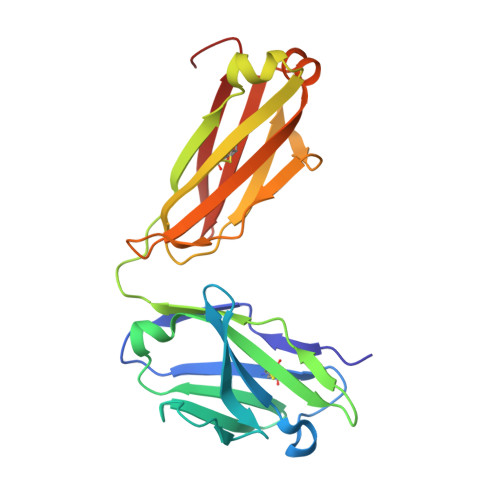
Structure of the Anti-C60 Fullerene Antibody Fab Fragment: Structural Determinants of Fullerene Binding.
Osipov, E.M., Hendrickson, O.D., Tikhonova, T.V., Zherdev, A.V., Solopova, O.N., Sveshnikov, P.G., Dzantiev, B.B., Popov, V.O.(2019) Acta Naturae 11: 58-65
- PubMed: 31024749
- Primary Citation of Related Structures:
6H3H - PubMed Abstract:
The structure of the anti-C 60 fullerene antibody Fab fragment (FabC 60 ) was solved by X-ray crystallography. The computer-aided docking of C 60 into the antigen-binding pocket of FabC 60 showed that binding of C 60 to FabC 60 is governed by the enthalpy and entropy; namely, by π-π stacking interactions with aromatic residues of the antigen-binding site and reduction of the solvent-accessible area of the hydrophobic surface of C 60 . A fragment of the mobile CDR H3 loop located on the surface of FabC 60 interferes with C 60 binding in the antigen-binding site, thereby resulting in low antibody affinity for C 60 . The structure of apo-FabC 60 has been deposited with pdbid 6H3H.
- Bach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Leninsky Ave. 33, 119071, Moscow, Russia.
Organizational Affiliation: