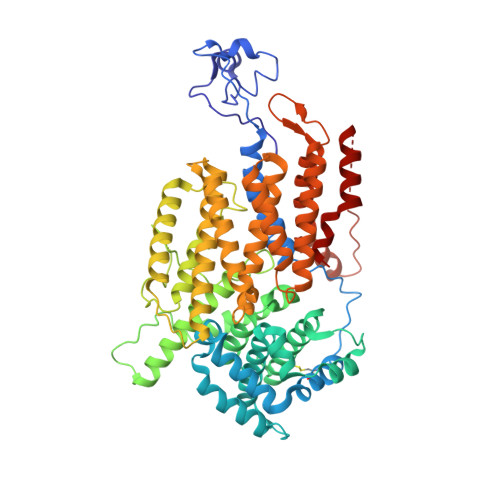
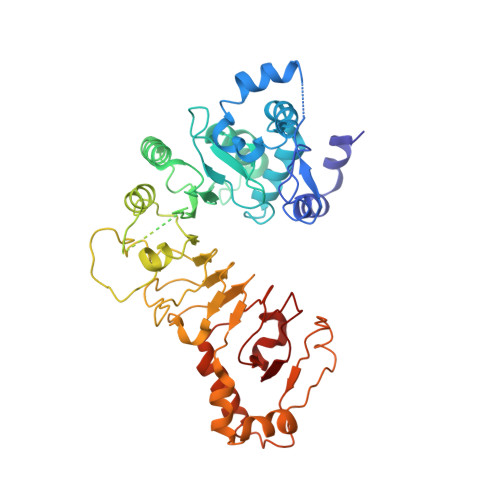
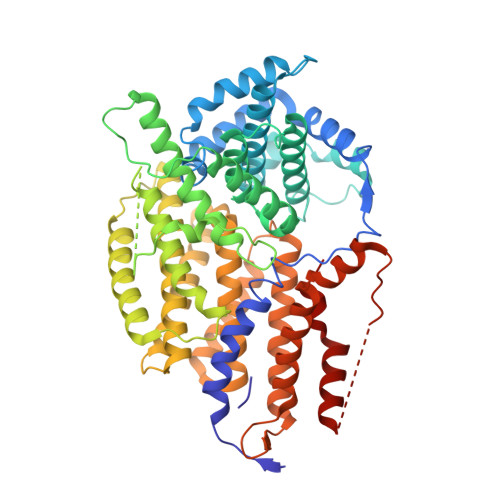
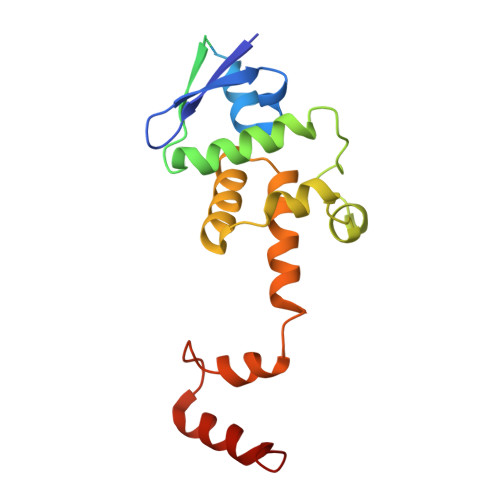
Architecture of the CBF3-centromere complex of the budding yeast kinetochore.
Yan, K., Zhang, Z., Yang, J., McLaughlin, S.H., Barford, D.(2018) Nat Struct Mol Biol 25: 1103-1110
- PubMed: 30478265
- DOI: https://doi.org/10.1038/s41594-018-0154-1
- Primary Citation of Related Structures:
6GYP, 6GYS, 6GYU - PubMed Abstract:
Kinetochores are multicomponent complexes responsible for coordinating the attachment of centromeric DNA to mitotic-spindle microtubules. The point centromeres of budding yeast are organized into three centromeric determining elements (CDEs), and are associated with the centromere-specific nucleosome Cse4. Deposition of Cse4 at CEN loci is dependent on the CBF3 complex that engages CDEIII to direct Cse4 nucleosomes to CDEII. To understand how CBF3 recognizes CDEIII and positions Cse4, we determined a cryo-EM structure of a CBF3-CEN complex. CBF3 interacts with CEN DNA as a head-to-head dimer that includes the whole of CDEIII and immediate 3' regions. Specific CEN-binding of CBF3 is mediated by a Cep3 subunit of one of the CBF3 protomers that forms major groove interactions with the conserved and essential CCG and TGT motifs of CDEIII. We propose a model for a CBF3-Cse4-CEN complex with implications for understanding CBF3-directed deposition of the Cse4 nucleosome at CEN loci.
- MRC Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: