Contribution of a Multifunctional Polymerase Region of Foot-and-Mouth Disease Virus to Lethal Mutagenesis.
de la Higuera, I., Ferrer-Orta, C., Moreno, E., de Avila, A.I., Soria, M.E., Singh, K., Caridi, F., Sobrino, F., Sarafianos, S.G., Perales, C., Verdaguer, N., Domingo, E.(2018) J Virol 92
- PubMed: 30068642
- DOI: https://doi.org/10.1128/JVI.01119-18
- Primary Citation of Related Structures:
6GVV, 6GVY - PubMed Abstract:
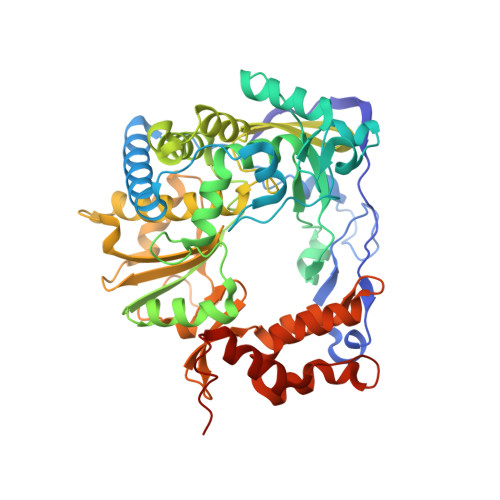
Viral RNA-dependent RNA polymerases (RdRps) are major determinants of high mutation rates and generation of mutant spectra that mediate RNA virus adaptability. The RdRp of the picornavirus foot-and-mouth disease virus (FMDV), termed 3D, is a multifunctional protein that includes a nuclear localization signal (NLS) in its N-terminal region. Previous studies documented that some amino acid substitutions within the NLS altered nucleotide recognition and enhanced the incorporation of the mutagenic purine analogue ribavirin in viral RNA, but the mutants tested were not viable and their response to lethal mutagenesis could not be studied. Here we demonstrate that NLS amino acid substitution M16A of FMDV serotype C does not affect infectious virus production but accelerates ribavirin-mediated virus extinction. The mutant 3D displays polymerase activity, RNA binding, and copying processivity that are similar to those of the wild-type enzyme but shows increased ribavirin-triphosphate incorporation. Crystal structures of the mutant 3D in the apo and RNA-bound forms reveal an expansion of the template entry channel due to the replacement of the bulky Met by Ala. This is a major difference with other 3D mutants with altered nucleotide analogue recognition. Remarkably, two distinct loop β9-α11 conformations distinguish 3Ds that exhibit higher or lower ribavirin incorporation than the wild-type enzyme. This difference identifies a specific molecular determinant of ribavirin sensitivity of FMDV. Comparison of several polymerase mutants indicates that different domains of the molecule can modify nucleotide recognition and response to lethal mutagenesis. The connection of this observation with current views on quasispecies adaptability is discussed. IMPORTANCE The nuclear localization signal (NLS) of the foot-and-mouth disease virus (FMDV) polymerase includes residues that modulate the sensitivity to mutagenic agents. Here we have described a viable NLS mutant with an amino acid replacement that facilitates virus extinction by ribavirin. The corresponding polymerase shows increased incorporation of ribavirin triphosphate and local structural modifications that implicate the template entry channel. Specifically, comparison of the structures of ribavirin-sensitive and ribavirin-resistant FMDV polymerases has identified loop β9-α11 conformation as a determinant of sensitivity to ribavirin mutagenesis.
- Centro de Biología Molecular "Severo Ochoa" (CSIC-UAM), Cantoblanco, Madrid, Spain.
Organizational Affiliation: