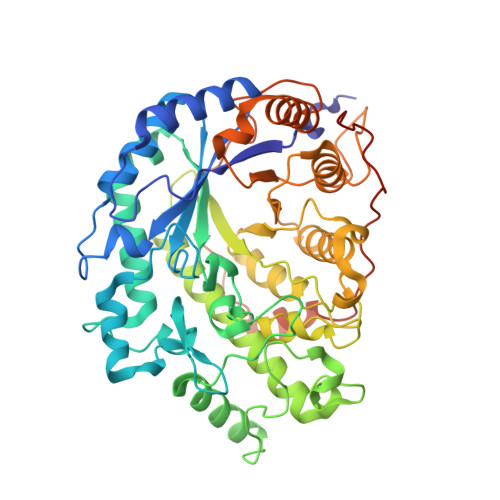
Three-dimensional structure of the wheat beta-amylase Tri a 17, a clinically relevant food allergen.
Hofer, G., Wieser, S., Bogdos, M.K., Gattinger, P., Nakamura, R., Ebisawa, M., Makela, M., Papadopoulos, N., Valenta, R., Keller, W.(2019) Allergy 74: 1009-1013
- PubMed: 30515829
- DOI: https://doi.org/10.1111/all.13696
- Primary Citation of Related Structures:
6GER - Institute of Molecular Biosciences, BioTechMed Graz, University of Graz, Graz, Austria.
Organizational Affiliation: